Nodal slow pathway ablation is the treatment of choice for nodal reentrant tachycardia. No demographic, anatomic, or electrophysiologic variables have been reported to predict an exact location of the slow pathway in the atrioventricular node or its proximity to the fast pathway. The purpose of this study was to analyze these variables.
MethodsThe study prospectively included 54 patients (17 men; mean age, 55 [16] years) who had undergone successful slow pathway ablation. The refractory periods of both pathways and their differential conduction time were measured, and calculations were performed to obtain the distance from the His-bundle region (location of the fast pathway) to the coronary sinus ostium (to estimate the anteroposterior length of the triangle of Koch) and to the slow pathway area.
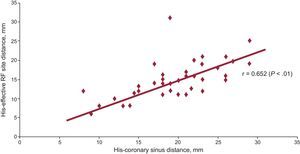
ResultsThe differential conduction time (139 [98] ms) did not correlate with the His-coronary sinus distance (19 [6] mm; P=.6) or the His-slow pathway distance (14 [4] mm; P=.4). When the His-coronary sinus distance was larger, the His-slow pathway distance was also larger (r=0.652; P<.01) and the anatomic correlation between the triangle dimensions and the separation between the two pathways was confirmed. In patients older than 70 years, smaller triangle sizes and a shorter distance between both pathways were observed (P<.001).
ConclusionsA greater anteroposterior dimension of the triangle of Koch is associated with a slow-pathway location farther from the fast pathway. In elderly patients the two pathways are closer together (higher risk of atrioventricular block).
Keywords
Nodal reentrant tachycardia (NRT) is the most common form of presentation of paroxysmal supraventricular tachycardia.1 The electrophysiologic mechanism behind NRT initiation and maintenance is reentry between 2 pathways with different conduction characteristics, both of which are part of the atrioventricular nodal (AVN) structure.1,2 The slow pathway (SP) is identified as an inferior and posterior AVN extension near the coronary sinus ostium (CSO), which is anatomically the inferior apex of the triangle of Koch.2–4 Nevertheless, a clear histologic differentiation between the 2 pathways (slow and fast) in the AVN is still controversial.2,5–8 The unpredictable anatomic separation between the SP and the fast pathway (FP) plays a critical role in the outcome of SP ablation (the treatment of choice in these patients), due to the possible risk of atrioventricular (AV) block caused by FP involvement.9,10
Previous studies have suggested that the anatomic location of the SP may correlate with its electrophysiologic properties.11,12 These properties include the duration of the A(H)-A(Md) interval, which would express the “electrical” distance between the 2 pathways and would indicate the risk of AV block during SP ablation.13 However, no linear correlation has been identified between conduction times and FP and SP refractory periods or their relative anatomic situation. There are also no data on how patient age at NRT onset influences the anatomic site of SP, its proximity to the FP or, therefore, the risk of AV block during ablation.
Lastly, no studies have investigated the influence of more than 2 conduction patterns in the AVN (demonstrated by the presence of a second jump of the atrial-His [AH] interval, seen in up to 40% of NRT cases) on SP ablation outcome.14
Our purpose was to identify whether there is a correlation between SP and FP electrophysiologic characteristics, triangle of Koch size, and SP location in patients with NRT. We also analyzed the influence of sex and age on SP location.
METHODSStudy PopulationWe prospectively included 56 patients who had undergone electrophysiologic analysis and successful NRT ablation. All patients gave written consent in accordance with our institutional guidelines. To determine the exact anatomic location of SP in all patients, we excluded any patients with other mechanisms of supraventricular tachycardia or with unsuccessful ablation.
Electrophysiologic StudyThe electrophysiologic study was performed through the right femoral vein, advancing 2 electrode catheters (tetrapolar, 6 Fr, with electrodes spaced 5 mm apart, Bard Inc.; Murray Hill, New Jersey, United States) to positions in the upper right atrium and His/right ventricle.
Intracardiac bipolar electrograms (filter at 30-500Hz) were recorded and saved throughout the procedure using the Lab Pro system (Bard Inc.) and were subsequently analyzed. Electronic calibrators with a 2-ms resolution were used at a screen speed of 100 mm/s for all measurements. The NRT diagnosis was confirmed using previously described electrophysiologic criteria, including criteria for para-Hisian entrainment without His-bundle capture.1,15
Electrophysiologic MeasurementsThe electrophysiologic measurements included the refractory periods of both nodal pathways, sinus cycle length, tachycardia cycle, AH- and His-ventricle intervals, and antegrade Wenckebach point, before and after ablation. Dual nodal pathway physiology (presence of nodal SP) was established if an increase > 50 ms in the AH interval (A2H2) was observed along with a decrease of 10 ms in the coupling interval during elective atrial pacing (AH jump). The conduction time difference between SP and FP was measured as the difference between the A2H2 interval in the first beat after the AH jump (initial SP conduction) and the maximum A2H2 interval before reaching the FP effective refractory period (as an approximation of the longest FP conduction time before the AH jump). Unlike previous reports, we speculated that this conduction time difference would better express the differential conduction characteristics of the 2 pathways, unlike the individual AH-interval measurements during FP or SP conduction.11,12 A second AH jump is expressed as A3H3.
Anatomic Measurements of the Triangle of KochThe anatomic measurements were performed using fluoroscopic calibrators with the 5-mm reference in the electrode distance of 1 of the tetrapolar leads (His-bundle lead in right anterior oblique view; right atrial lead in left anterior oblique view). To prevent errors in the measurements related to more vertical or horizontal heart orientation, both views were adjusted to obtain an exact lead-to-lead measurement of 5 mm in the reference catheter.16–19
Before the ablation procedure was started, the mapping/ablation catheter was advanced to the coronary sinus until the proximal leads were at the level of the CSO. The distance (in millimeters) between the CSO and the proximal His-bundle region was then measured in the left anterior oblique view (view with best catheter deployment in the coronary sinus, with the CSO indicating the lower limit of the triangle of Koch). Therefore, the His-CSO distance was estimated as an approximation of the vertical length of the triangle of Koch. By dividing this length into 3 thirds, the SP location was qualitatively classified into a high, middle, or low position. Following ablation, the FP-to-SP distance was determined by using the right anterior oblique views and measuring the distance between the distal lead of the mapping/ablation catheter (region of effective SP ablation) and the proximal recording region of the His bundle.
Slow Pathway AblationThe radiofrequency energy was 50 W (55°C) in the SP area, when a solid-tip 4-mm ablation catheter was used (electrode distance, 5 mm; Bard Inc.). Radiofrequency emission was discontinued when AV dissociation was observed during AV junctional (nodal) rhythm or after 30 s to 60 s of application with sufficient nodal rhythm.18 The procedure was considered successful if NRT could not be induced by elective atrial pacing during a 20-min observation period (during which isoproterenol was administered in intravenous perfusion) and if there were no observations of ≥ 2 beats due to nodal reentry. The final effective SP ablation location was considered to be the last radiofrequency application site after which NRT could not be reinduced.
Influence of Age and Sex on the Pathophysiology of Nodal Reentry TachycardiaIf a significant correlation is found between SP anatomic position and demographic characteristics, a different pathophysiology of nodal reentry based on age or sex could be concluded in patients with NRT.20 To confirm this hypothetical finding, it was considered necessary to supplement this analysis with a retrospective study of a separate cohort of 325 consecutive patients who had undergone successful SP ablation at our hospital, with the population divided into different age and/or sex brackets.
Statistical AnalysisThe categorical variables are expressed as frequency and percentage, and the quantitative variables as mean (standard deviation) or median [interquartile range]. Potential associations between quantitative and categorical variables were analyzed by the Student t test (for independent measurements in the case of 2 categories) or by analysis of variance and the Tukey range test (for multiple comparisons in the case of variables with > 2 categories). The relationship between 2 quantitative variables was measured by the Pearson correlation coefficient, expressing r and the significance level. In all analyses, a P value<.05 was considered statistically significant. The IBM SPSS 19.0 statistical package (IBM Corp.; New York, United States) was used for the statistical analysis.
ResultsStudy PopulationA total of 2 of 56 patients were ultimately excluded from the analysis due to unsuccessful SP ablation. Among the remaining 54 patients, 17 were men and the mean age was 55 (16) years. Structural heart disease was documented in 7 patients: ischemic heart disease in 3, and hypertensive heart disease in the remaining 4.
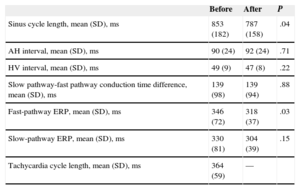
Electrophysiological Findings and AblationThere were no significant differences in baseline parameters for AV conduction or the FP conduction characteristics before and after ablation, except for a shorter FP effective refractory period after ablation (P=.03) (Table 1).
Electrophysiological Parameters Before and After Effective Ablation of the Nodal Slow Pathway
| Before | After | P | |
|---|---|---|---|
| Sinus cycle length, mean (SD), ms | 853 (182) | 787 (158) | .04 |
| AH interval, mean (SD), ms | 90 (24) | 92 (24) | .71 |
| HV interval, mean (SD), ms | 49 (9) | 47 (8) | .22 |
| Slow pathway-fast pathway conduction time difference, mean (SD), ms | 139 (98) | 139 (94) | .88 |
| Fast-pathway ERP, mean (SD), ms | 346 (72) | 318 (37) | .03 |
| Slow-pathway ERP, mean (SD), ms | 330 (81) | 304 (39) | .15 |
| Tachycardia cycle length, mean (SD), ms | 364 (59) | — |
AH, atrial-His; ERP, effective refractory period; HV, His-ventricle; SD, standard deviation.
Data are expressed as mean (standard deviation). P < .05 was considered statistically significant.
Although tachycardia tended to be slower (longer cycle length) when the differential SP-FP conduction time was longer, this difference was not statistically significant overall (P=.058) or in any age group.
The SP-FP conduction time difference was 139 (98) ms, with considerable variability in absolute values (50-376 ms). This high variability was attributed to a wide range of SP conduction times.
In 6 patients, only 1 application of successful radiofrequency was performed; all other patients required > 1 radiofrequency application.
Triangle of Koch Anatomy and Electrophysiologic Characteristics of the Slow PathwayThe His-CSO distance was 19 (6) mm and the His-SP distance, 14 (4) mm. The SP-FP conduction time difference did not correlate with His-CSO distance (r=–0.084; P=.6) or His-SP distance (r=–0.138; P=.4). In contrast, triangle of Koch size (ie, His-CSO distance) correlated with a more caudal SP location in the AVN structure (His-SP distance): r=0.652 (P<.01) (Figure 1).
Positive correlation between the triangle of Koch size (His-coronary sinus ostium distance) and effective radiofrequency site (slow pathway location). When the His-coronary sinus ostium distance is longer, a more caudal location of the slow pathway is observed. RF, radiofrequency.
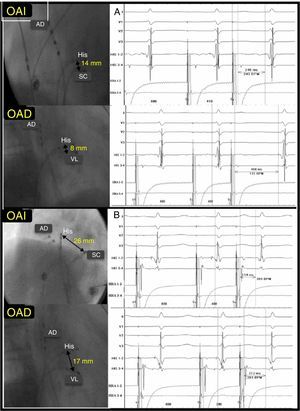
These results suggest that FP-SP distance is longer when the triangle of Koch is larger, although the conduction characteristics of both pathways remain unaffected (Figure 2).
Correlation between His-coronary sinus ostium to His-slow pathway distance with no correlation with slow pathway-fast pathway conduction time difference. A, short His-coronary sinus ostium distance and significant SP-FP differential conduction time (458 – 246=212 ms). B, long His-coronary sinus ostium distance and minimal SP-FP differential conduction time (312 – 254=58 ms). CSO, coronary sinus ostium; FP, fast pathway; LAOP, left anterior oblique projection; RA, right atrium; RAOP, right anterior oblique projection; SP, slow pathway.
A total of 18 patients (33%) had a second AH jump; the mean A3H3 increase was 103 (77) ms. A second AH jump was not associated with any specific FP or SP conduction properties or with any particular age or sex subgroup. The His-CSO and His-SP distances were also no different in the presence of a second AH jump compared with those in other patients (19 [8] vs 20 [8] mm; P=.4 and 14 (4) vs 15 (5) mm; P=.5).
The number of radiofrequency applications required to achieve successful SP ablation also did not differ when a second jump was observed (9 [8] vs 6 [4] pulses; P=.3).
Demographic Characteristics and Slow Pathway LocationA statistical correlation was established between young age and longer His-SP distance (P<.001). Younger patients had a larger SP-FP conduction time difference (due to longer SP conduction time; r=–0.341; P=.014), with no sex-related differences (P=.71).
Only young patients showed a very weak electrophysiologic-anatomic correlation between slower SP conduction time (A2H2 at first beat after AH jump) and more caudal location rate (r=–0.317; P=.046). However, the SP-FP conduction time difference was not useful in establishing any correlations between the electrophysiologic characteristics of both nodal pathways and the triangle of Koch anatomy in any age group.
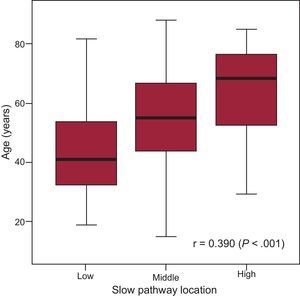
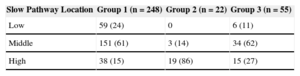
In our retrospective cohort of 325 patients (65%, women; median age, 54 [41-69] years), the successful SP ablation site was qualitatively classified as high in 72 patients, middle in 188, and low in 65. A linear correlation between SP location and aging was observed, and a high SP location was associated with advanced age (r=0.390; P<.001) (Figure 3), particularly in patients > 70 years with recent-onset NRT (< 12 months; P<.001) (Table 2). There were no sex-related differences among the various groups WITH high, middle, or low SP location.
Age-related Slow Pathway (Low, Medium, or High) vs Fast Pathway Location. Analysis of a Retrospective Cohort of 325 Patients
| Slow Pathway Location | Group 1 (n=248) | Group 2 (n=22) | Group 3 (n=55) |
|---|---|---|---|
| Low | 59 (24) | 0 | 6 (11) |
| Middle | 151 (61) | 3 (14) | 34 (62) |
| High | 38 (15) | 19 (86) | 15 (27) |
Data are expressed as No. (%).
Group 2 vs groups 1 and 3, P<.001.
Group 1, age<70 years; group 2, age > 70 years with recent onset (< 12 months) of paroxysmal nodal reentrant tachycardia; group 3, age > 70 years with nodal reentrant tachycardia of prior onset (> 12 months).
This study observed a positive correlation between the anatomic size of the triangle of Koch (expressed as His-CSO distance) and the SP effective ablation site (His-SP distance) in patients with NRT. Additionally, young age was associated with a lower SP location (and, therefore, with a lower risk of AV block during ablation). However, our study confirmed the absence of a clear electrophysiologic-anatomic correlation between the FP and SP conduction properties and the triangle of Koch dimensions or the relative SP-FP location. Accordingly, SP conduction properties are not predictive of a specific anatomic SP position compared with the FP in the triangle of Koch and do not provide an anatomic guide for SP ablation. We also believe that the sole finding of a correlation between SP conduction velocity and anatomic site compared with FP in young patients should be analyzed with caution. No anatomic-electrophysiologic correlation was shown in this age group when the electrophysiologic variable used was SP-FP conduction difference, which, in our opinion, was more demonstrative of the differential electrophysiologic characteristics between the 2 pathways.
We believe that the finding of a strong correlation between advanced age at NRT onset and high SP location (P<.001) could indicate a different age-related NRT pathophysiology, which would specifically affect the SP location and its proximity to the FP. Lastly, our work showed that a second AH jump had no substantial clinical implications for NRT ablation.
Factors That Influence the Slow Pathway Site: Different Age-Related Pathophysiology for Nodal Reentrant Tachycardia?Unlike our study, Manolis et al21 analyzed a series of 55 patients with NRT but found no correlation between age and any specific SP sites. Sanchez-Quintana et al6 reported on 16 hearts examined during autopsy, which showed the presence of at least 2 histologic and anatomic SP positions: a) a more posterior and inferior localization, in which the SP emerges from nonspecialized atrial tissue when the muscle fibers are closer to this specific region of the AVN, and b) a middle or high location, more cephalic to the CSO (and, therefore, potentially nearer the FP). In this last scenario, the orientation of the muscle fibers and the surrounding connective tissue (microfibrosis) would contribute to the initiation of ≥ 2 different AVN conduction patterns, as a result of a variable degree of nonuniform anisotropy, which would allow reentry. Consequently, NRT can occur, despite the absence of 2 histologically differentiable pathways within the AVN. A background of postinflammatory and/or degenerative microfibrosis limited to the anterior portion of the AVN (more similar to the latter histologic pattern) has been specifically linked to an elderly NRT population.22 Our observation of a short His-SP distance in patients older than 70 years with recent-onset NRT is consistent with this finding, as both pathways would be very close to each other under these circumstances and located in the anterior portion of the AVN. In contrast, an increased CSO width (which results in longer His-CSO and His-SP distances) has been related to a more apparent (histologic) and pronounced posteroinferior AVN extension.20,22,23 Our analysis found a link between this different anatomic and histologic substrate of the SP position compared with the FP in a younger population. We feel that these findings are consistent with a different age-related NRT pathophysiology, probably with distinct histopathologic patterns. However, our study was unable to confirm this assumption due to a lack of histologic data.
Electrophysiologic Characteristics of Slow Pathway and Triangle of Koch AnatomyIn a series published by Geller et al,11 the AH interval during SP conduction correlated with the distance between the SP ablation site and the His bundle, but not with the total size of the triangle of Koch. In our series, we found no anatomic-electrophysiologic correlation between SP-FP proximity or conduction properties, using the SP-FP conduction time difference as a reference. This variable was considered a more reliable electrophysiologic function, as it considers SP vs FP conduction properties. The absolute value of the AH interval during SP conduction is highly variable and influenced by multiple factors, mainly interpatient and intraprocedure variability in vagal tone. This can lead to dramatic changes in SP conduction time (expressed as AH interval) and jeopardize the validity of comparisons between the anatomic and electrophysiologic characteristics of the SP. Our study considered that a comparison of FP and SP conduction properties at the same time point in the electrophysiologic study would be more reliable when establishing a possible (and a posteriori, unproven) anatomic-electrophysiologic correlation.
Triangle of Koch AnatomyThe triangle of Koch dimensions obtained using the His-CSO distance measurement was somewhat shorter in our study population than in other series.7,17 Nevertheless, McGuire et al19 reported similar results from an analysis of postmortem and postoperative samples that probably more accurately represent the true anatomic dimensions of the triangle. This fact appears to validate the use of the His-CSO fluoroscopic distance as a measurement of the cephalocaudal length of the triangle of Koch. The His-SP distance (14 [4] mm in our series) was similar to the distance reported in other series (13-16 mm).5,7,9
Earlier studies have demonstrated wide variability in triangle of Koch dimensions, with no clear correlation between the triangle length and the SP location.12,17,19 In contrast, we observed a correlation between triangle of Koch size and a specific SP location. This discrepancy can be explained by the concurrence of various anatomic and histologic SP positions among the various study populations. As mentioned earlier, these different pathophysiologic substrates of NRT were age-related. Therefore, we believe that the analysis of a more homogeneous population in terms of age groups would confirm a positive correlation between the His-CSO and His-SP distances.
LimitationsThe triangle of Koch is a 3-dimensional structure; hence, the 2-dimensional measurements obtained in this study do not represent the true anatomic dimensions of this structure with absolute precision. Additionally, these values may vary during the different phases of the cardiac cycle and/or respiratory movements. However, the similar results obtained by direct anatomic observation of the triangle of Koch seem to validate our methodology.19 The ablation catheter position in the coronary sinus was determined using standard criteria: a) anatomic, using both oblique views; b) interpretation of intracardiac electrograms, and c) observation of increased impedance. No venography techniques were used.
Our series observed no episodes of persistent AV block after ablation and, therefore, our study cannot be used to confirm a correlation between the highest SP location and a higher risk of AV block. In view of the anatomic correlations observed and associated with certain age groups, however, our analysis should be helpful in deciding on ablation strategies in terms of choosing the most adequate ablation catheter in each case and to anticipate a higher a priori risk of AV block among elderly patients.
Prior radiofrequency emission in lower and/or higher areas of the triangle of Koch with respect to the effective application area could be the source of errors in the determination of the exact SP location, due to an additive effect. In our understanding, this possible limitation was minimized by tachycardia reinduction after each application until effective application.
The measurements were not performed during autonomic blockade. Therefore, some influence of autonomic tone on the measurements cannot be ruled out entirely, as previously discussed.24,25 The analysis did not exclude patients who required isoproterenol infusion for tachycardia induction, although its use may influence the conduction time for both pathways.12 We believe that this disadvantage is minimized by the use of a relative value (SP-FP conduction time difference) rather than an absolute value for the AH jump.
Finally, the retrospective study arbitrarily set a threshold of 70 years as it considered that at advanced ages, the NRT mechanism could be due to tissue degeneration in the AVN structure and not to the presence of 2 histologically and/or anatomically differentiated nodal pathways, particularly in patients with recent onset (< 12 months) of tachyarrhythmia; naturally, this is purely speculative and should be confirmed by another type of study.
CONCLUSIONSA more caudal, and, therefore, safer location for the effective SP ablation site is associated with a longer longitudinal dimension of the triangle of Koch and can be anticipated in patients aged < 70 years. No correlation between the anatomic and electrophysiologic characteristics of the 2 nodal pathways has been shown to exist. These results actually generate hypotheses and should be confirmed in further studies, in view of the important strategic implications for SP ablation.
CONFLICTS OF INTERESTNone declared.