In patients with heart failure and reduced ejection fraction (HFrEF), several therapies have been proven to reduce mortality in clinical trials. However, there are few data on the effect of the use of evidence-based therapies on causes of death in clinical practice.
MethodsThis study included 2351 outpatients with HFrEF (< 40%) from 2 multicenter prospective registries: MUSIC (n=641, period: 2003-2004) and REDINSCOR I (n=1710, period: 2007-2011). Variables were recorded at inclusion and all patients were followed-up for 4 years. Causes of death were validated by an independent committee.
ResultsPatients in REDINSCOR I more frequently received beta-blockers (85% vs 71%; P <.001), mineralocorticoid antagonists (64% vs 44%; P <.001), implantable cardioverter-defibrillators (19% vs 2%; P <.001), and resynchronization therapy (7.2% vs 4.8%; P=.04). In these patients, sudden cardiac death was less frequent than in those in MUSIC (6.8% vs 11.4%; P <.001). After propensity score matching, we obtained 2 comparable populations differing only in treatments (575 vs 575 patients). In patients in REDINSCOR I, we found a lower risk of total mortality (HR, 0.70; 95%CI, 0.57-0.87; P=.001) and sudden cardiac death (sHR, 0.46; 95%CI, 0.30-0.70; P <.001), and a trend toward lower mortality due to end-stage HF (sHR, 0.73; 95%CI, 0.53-1.01; P=.059), without differences in other causes of death (sHR, 1.17; 95%CI, 0.78-1.75; P=.445), regardless of functional class.
ConclusionsIn ambulatory patients with HFrEF, implementation of evidence-based therapies was associated with a lower risk of death, mainly due to a significant reduction in sudden cardiac death.
Keywords
Current therapeutic care of patients with acute heart failure (HF) is based on left ventricular ejection fraction (LVEF). In patients with reduced LVEF (<40%), drug-based neurohormonal blocking and the use of devices have been proven to have well-established beneficial effects on mortality.1,2 Additionally, beta-blockers and implantable cardioverter-defibrillators (ICDs) have been specifically shown to reduce sudden cardiac death, whereas angiotensin-converting enzyme inhibitors (ACEIs) (or angiotensin II receptor blockers), mineralocorticoid antagonists, and cardiac resynchronization therapy mainly reduce mortality due to HF.3–7 It has recently been observed that neprilysin and angiotensin-receptor inhibition reduce all-cause mortality, including cardiovascular, HF, and sudden cardiac death.8
Although these therapies are clearly recommended in clinical guidelines, inclusion in clinical practice has been slow. Indeed, various European and Spanish registries have shown that these drugs are underprescribed in clinical practice.9–11 This inadequate use of therapies known to be beneficial is attributable, at least in part, to differences between clinical trial participants and patients in real-world clinical practice, making it hard to extend the benefits observed in randomized trials to the clinical setting. In Spain, the MUSIC and REDINSCOR I multicenter registries were conducted with outpatients and investigated mortality, classifying it by cause.12–14 Recently, a time-based analysis of various clinical trials showed that the incidence of sudden cardiac death in the control groups has declined in more recent trials, a finding attributed to improvements in drug therapies.15 However, there are no clinical practice data on the effect of including treatments with prognostic benefits on the various causes of death.
The aim of this study was to evaluate the effects of evidence-based treatments on mortality and on the various causes of death in real-world clinical practice populations.
METHODSStudy population and designA total of 2351 patients with LVEF <40% were included in 2 prospective cohorts from 2 multicenter longitudinal registries of outpatients with chronic HF. The MUSIC registry was designed to evaluate predictors of the risk of cardiac mortality and sudden cardiac death.12 This registry included 992 consecutive outpatients who came to specialized HF clinics at 8 Spanish teaching hospitals between April 2003 and December 2004. All patients had chronic symptomatic HF (New York Heart Association [NYHA] functional classes II-IV) and were treated according to current recommendations. The REDINSCOR I registry13 was designed to evaluate the predictors of cardiac mortality and hospitalizations. A total of 2507 consecutive outpatients were selected between January 2007 and January 2011 in the HF units of 18 hospitals; 6 of these hospitals had participated in the previous registry. This study included only patients with LVEF <40% (641 patients in the MUSIC registry and 1710 patients in REDINSCOR I), as determined by echocardiography at the time of inclusion. All patients were symptomatic (NYHA II-IV), ambulatory, and received treatments optimized by the attending physician according to current clinical guidelines. In both registries, patients were excluded if they had severe valve disease eligible for surgical repair or had any other concomitant terminal disease. Both cohorts met the requirements of the Declaration of Helsinki, the protocols were approved by the ethics committees at each participating site, and all patients gave written informed consent.
Study variablesThe data were collected prospectively using an online database specifically designed for both registries, and were checked monthly for quality control.13,14 For the purposes of this study, the variables used were those collected in both registries with identical definitions. The baseline variables were recorded when the patient was enrolled and included demographic data, medical history, symptoms and physical examination, electrocardiogram, echocardiography, blood tests, and treatments at enrollment (table 1). To define each variable, identical standard criteria were used in each registry.12,13 Evidence-based treatments were considered to include angiotensin-converting enzyme inhibitors (or angiotensin II receptor antagonists), beta-blockers, mineralocorticoid antagonists, ICDs, and cardiac resynchronization therapy.
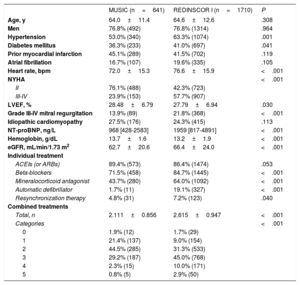
Baseline clinical characteristics of the 2 population cohorts compared
| MUSIC (n=641) | REDINSCOR I (n=1710) | P | |
|---|---|---|---|
| Age, y | 64.0±11.4 | 64.6±12.6 | .308 |
| Men | 76.8% (492) | 76.8% (1314) | .964 |
| Hypertension | 53.0% (340) | 63.3% (1074) | .001 |
| Diabetes mellitus | 36.3% (233) | 41.0% (697) | .041 |
| Prior myocardial infarction | 45.1% (289) | 41.5% (702) | .119 |
| Atrial fibrillation | 16.7% (107) | 19.6% (335) | .105 |
| Heart rate, bpm | 72.0±15.3 | 76.6±15.9 | <.001 |
| NYHA | <.001 | ||
| II | 76.1% (488) | 42.3% (723) | |
| III-IV | 23.9% (153) | 57.7% (907) | |
| LVEF, % | 28.48±6.79 | 27.79±6.94 | .030 |
| Grade III-IV mitral regurgitation | 13.9% (89) | 21.8% (368) | <.001 |
| Idiopathic cardiomyopathy | 27.5% (176) | 24.3% (415) | .113 |
| NT-proBNP, ng/L | 968 [428-2583] | 1959 [817-4891] | <.001 |
| Hemoglobin, g/dL | 13.7±1.6 | 13.2±1.9 | <.001 |
| eGFR, mL/min/1.73 m2 | 62.7±20.6 | 66.4±24.0 | <.001 |
| Individual treatment | |||
| ACEIs (or ARBs) | 89.4% (573) | 86.4% (1474) | .053 |
| Beta-blockers | 71.5% (458) | 84.7% (1445) | <.001 |
| Mineralocorticoid antagonist | 43.7% (280) | 64.0% (1092) | <.001 |
| Automatic defibrillator | 1.7% (11) | 19.1% (327) | <.001 |
| Resynchronization therapy | 4.8% (31) | 7.2% (123) | .040 |
| Combined treatments | |||
| Total, n | 2.111±0.856 | 2.615±0.947 | <.001 |
| Categories | <.001 | ||
| 0 | 1.9% (12) | 1.7% (29) | |
| 1 | 21.4% (137) | 9.0% (154) | |
| 2 | 44.5% (285) | 31.3% (533) | |
| 3 | 29.2% (187) | 45.0% (768) | |
| 4 | 2.3% (15) | 10.0% (171) | |
| 5 | 0.8% (5) | 2.9% (50) | |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor antagonists; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT–proBNP, N-terminal pro–brain natriuretic peptide; NYHA, New York Heart Association.
Data are expressed as % (No.), mean±standard deviation, or median [interquartile range].
Outpatient follow-up visits were performed every 6 to 12 months, for a median of 43 [29-46] months in the MUSIC registry and 40 [18-56] months in the REDINSCOR I registry. After combination of the 2 registries, follow-up was cut off at 48 months (4 years) in both cases. Six patients were lost to follow-up in the MUSIC registry and 11 patients in REDINSCOR I, and all were censored in the survival analysis. Information on patient deaths was collected from medical and administrative records. In each specific case, the cause of death was identified, in order to divide all-cause mortality into noncardiovascular and cardiovascular, with the latter further classified into death due to end-stage HF (including refractory HF and need for heart transplant), sudden cardiac death, or other cardiovascular death, according to previously established definitions.12–14 In all cases, the study investigators provided details on the death event, and the data were reviewed by an independent committee to validate the cause of death.
Statistical analysisQuantitative variables are expressed as the mean±standard deviation or median [interquartile range] (as applicable), and categorical variables as the percentage (number). The chi-square or Fisher exact test was used to compare qualitative variables, and the analysis of variance (ANOVA), Student t test, or Wilcoxon test was used for quantitative variables. Due to the nonrandomized nature of the study, propensity score matching (MatchIt, SPSS statistical package in R) was used to ensure comparable populations despite the different time periods and sources and to minimize any biases due to differences in clinical characteristics that could influence the prognosis.16 The characteristics of the propensity score matching model were as follows: a) 1:1 protocol without replacement; b) caliper width of ≤ 0.2 standard deviations of logit of propensity score; and c) “K-nearest neighbor matching” method for consistency. To evaluate between-group balance, the “difference in standardized means” was used to compare continuous and binary variables. This method is not influenced by sample size and allows variables with different units to be compared.17 In our case, standardized differences <0.2 were expected. Except for treatment variables (the study objective), all variables were used: age, sex, hypertension, diabetes, prior myocardial infarction, atrial fibrillation, heart rate, NYHA, LVEF, grade III-IV mitral regurgitation, ischemic etiology, N-terminal pro–brain natriuretic peptide [NT-proBNP]), hemoglobin, and glomerular filtration rate. Following this analysis, 2 groups of 575 patients were matched for inclusion in the MUSIC or REDINSCOR I registry, showing adequate overlap, as seen in figure 1 of the supplementary data. The survival analysis was performed for the entire population with LVEF <40% and for the groups resulting from propensity score matching. The Fine and Gray competing risks regression model was used to determine the effect of the explanatory variable (inclusion in 1 of the registries: MUSIC or REDINSCOR I) in the risk of each specific cause of death. Model calibration and discrimination were adequate (figure 2 of the supplementary data). The Schoenfeld residual test was used to assess the case of proportional subhazard ratio (sHR). Missing data were imputed using the Multivariate Imputation via Chained Equations (MICE) in R. Simple imputation (m=1) was performed because the percentage of missing data was <5% for all analytical variables, except for NT-proBNP, which was classified into 3 groups according to tercile (<750, 750-2414, or ≥2415ng/L) and as “data not available” (28.9%), because this parameter may not have been measured due to the event. The imputed group was the entire population available (n=2351). All statistical analyses were performed using SPSS 25 and R ver. 3.2. Statistical significance was set at a Pvalue <.05.
RESULTSStudy population and mortalityThe analysis included 2351 patients with LVEF <40% from the MUSIC (n=641, period: 2003-2004) and REDINSCOR I registries (n=1710, period: 2007-2011). The differences between the 2 registries are listed in table 1. In particular, patients from REDINSCOR I had a higher risk profile, with a higher percentage of diabetes, worse NYHA functional class, higher heart rate, lower LVEF, higher prevalence of mitral regurgitation, higher NT-proBNP concentration, and lower hemoglobin concentration. Only the estimated glomerular filtration rate was lower in the MUSIC population. There were no differences in age or sex or in the prevalence of atrial fibrillation or prior infarction. In terms of treatment, patients in REDINSCOR I had more optimized treatment, with considerable differences in the rates of beta-blockers, mineralocorticoid antagonists, and ICDs and a higher number of evidence-based combination treatments.
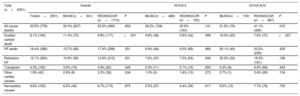
Mortality in each cohort at 48 months and the causes of death are listed in table 2. Total mortality was similar in both populations (33.0% vs 32.3%); however, the REDINSCOR I population exhibited differences in the distribution of causes as well as an effect due to the higher prevalence of advanced NYHA III-IV (58% vs 24%). Sudden cardiac death was less common in the more recent population (6.8% vs 11.4%; P<.001) and in both NYHA II (5.8% vs 9.8%; P=.009) and NYHA III-IV (7.6% vs 16.3%; P<.001) patients. The greater presence of advanced classes in the more recent population of REDINSCOR I led to a higher frequency of death due to end-stage HF in this population (16.4% vs 13.7%; P=.031); however, this difference disappeared after considering NYHA II (9.8% vs 9.5%; P=.866) and NYHA III-IV (23.2% vs 26.1%; P=.425) separately in both populations. The same effect was observed with other cardiovascular causes, which accounted for less than 2% in each population. Noncardiovascular mortality was similar between the 2 populations. Consequently, the relative contribution of sudden cardiac death to overall mortality was different in each population: sudden cardiac death accounted for 25.8% of deaths in NYHA II patients in REDINSCOR I vs 50.0% in MUSIC and for 18.5% of deaths in NYHA III-IV patients in REDINSCOR I vs 31.5% in MUSIC (P<.001).
Mortality at 48 months according to cause, functional class, and population cohort
| Total (n=2351) | Overall | NYHA II | NYHA III-IV | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total(n=2351) | MUSIC(n=641) | REDINSCOR I(n=1710) | P | MUSIC(n=488) | REDINSCOR I(n=723) | P | MUSIC(n=153) | REDINSCOR I(n=987) | P | |
| All-cause deaths | 33.0% (776) | 32.3% (207) | 33.3% (569) | .652 | 26.2% (128) | 22.5% (163) | .141 | 51.6% (79) | 41.1% (406) | .015 |
| Sudden cardiac death | 8.1% (190) | 11.4% (73) | 6.8% (117) | <.001 | 9.8% (48) | 5.8% (42) | .009 | 16.3% (25) | 7.6% (75) | <.001 |
| HF death | 16.4% (386) | 13.7% (88) | 17.4% (298) | .031 | 9.8% (48) | 9.5% (69) | .866 | 26.1% (40) | 23.2% (229) | .425 |
| Refractory HF | 12.1% (284) | 10.8% (69) | 12.6% (215) | .231 | 7.6% (37) | 7.5% (54) | .942 | 20.9% (32) | 16.3% (161) | .158 |
| Transplant | 4.3% (102) | 3.0% (19) | 4.9% (83) | .045 | 2.3% (11) | 2.1% (15) | .833 | 5.2% (8) | 6.9% (68) | .443 |
| Other cardiac causes | 1.9% (45) | 0.9% (6) | 2.3% (39) | .034 | 1.0% (5) | 1.8% (13) | .275 | 0.7% (1) | 2.6% (26) | .134 |
| Noncardiac causes | 6.6% (155) | 6.2% (40) | 6.7% (115) | .673 | 5.5% (27) | 5.4% (39) | .917 | 8.5% (13) | 7.7% (76) | .733 |
HF, heart failure; NYHA, New York Heart Association.
Data are expressed as % (No.).
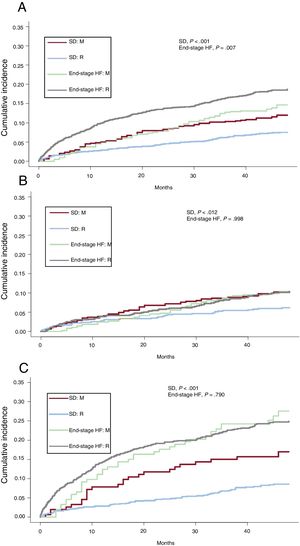
Figure 1 shows the cumulative incidence in the competing risks analysis of sudden death and death due to refractory HF, separated by functional class. The cumulative incidence of sudden death was lower in the more recent population of REDINSCOR I, yielding a significant reduction in the relative risk of the overall populations (sHR=0.61; 95% confidence interval [95%CI], 0.46-0.82; P<.001) as well as of patients in NYHA II (sHR=0.59; 95%CI, 0.39-0.90; P=.013) and in NYHA III-IV (sHR=0.47; 95%CI, 0.30-0.74; P=.001). Conversely, mortality due to end-stage HF was similar in the overall population (P=.081) in NYHA II (P=.964) or NYHA III-IV (P=.374).
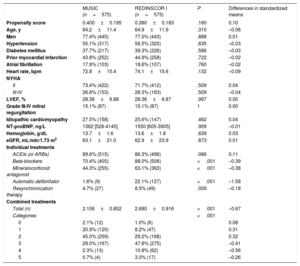
Propensity score matchingRisk propensity score matching was performed, obtaining 2 comparable populations in terms of risk factors and functional class (575 patients from MUSIC vs 575 patients from REDINSCOR I), which differed only in the treatments received (table 3). The more recent population received more optimized, evidence-based treatment, whether individual or combined therapies (P<.001). After this matching, as shown in figure 2, the overall REDINSCOR I population with more optimized treatment had a lower cumulative incidence of death (27.4% vs 36.5%; P=.001), as well as in NYHA II (21.8% vs 29.9%; P=.008) and NYHA III-IV (42.4% vs 53.9%; P=.047), mainly due to the significant decline in sudden cardiac death. The distribution of causes of death by category is shown in table 1 of the additional material. table 4 shows the decrease in the relative risk of the various causes of death, according to functional class. The lower mortality in the recent population was due to a lower risk of sudden cardiac death (sHR=0.46; 95%CI, 0.30-0.70; P<.001) whereas death due to end-stage HF showed a trend (sHR=0.73; 95%CI, 0.53-1.01; P=.059) and there were no differences in death due to other causes (sHR=1.17; 95%CI, 0.78-1.75; P=.445). The impact of the decrease in sudden cardiac death was stronger among patients in NYHA II, due to the higher relative contribution of sudden cardiac death to the total number of deaths in these patients: mortality was 36.7% in MUSIC vs 25.3% in REDINSCOR I for patients in NYHA II, and 31.6% in MUSIC vs 18.0% in REDINSCOR I for patients in NYHA III-IV. To confirm the impact of the various treatments, the population with cardiac resynchronization therapy and ICD was excluded using a stepwise approach. Once devices were excluded, the decrease was still observed in all-cause mortality and in sudden cardiac death associated with improved drug therapy in the more recent population (table 2 of the supplementary data and table 3 of the supplementary data). In the competing risks analysis of sudden cardiac death predictors, the presence of prior infarction, fewer than 2 evidence-based treatments, higher NT-proBNP, and lower estimated glomerular filtration rates were independent predictors in the overall population (C statistic=0.640) (table 4 of the supplementary data). None of the treatments were proven to be an independent predictor on their own, and no interactions were found between treatments. The use of 2 or more treatments was the therapeutic variable with the best sensitivity and significance in the analysis (table 5 of the supplementary data). An analysis of individual treatments in the matched population, through “MUSIC vs REDINSCOR I” interactions, revealed no statistically significant relationships (omnibus test, P=.320).
Comparison of baseline clinical characteristics of the MUSIC and REDINSCOR I populations in the risk-score matched populations
| MUSIC (n=575) | REDINSCOR I (n=575) | P | Differences in standardized means | |
|---|---|---|---|---|
| Propensity score | 0.400±0.195 | 0.380±0.183 | .160 | 0.10 |
| Age, y | 64.2±11.4 | 64.9±11.9 | .310 | –0.06 |
| Men | 77.4% (445) | 77.0% (443) | .888 | 0.01 |
| Hypertension | 55.1% (317) | 56.5% (325) | .635 | –0.03 |
| Diabetes mellitus | 37.7% (217) | 39.3% (226) | .586 | –0.03 |
| Prior myocardial infarction | 43.8% (252) | 44.9% (258) | .722 | –0.02 |
| Atrial fibrillation | 17.9% (103) | 18.6% (107) | .760 | –0.02 |
| Heart rate, bpm | 72.8±15.4 | 74.1±15.6 | .132 | –0.09 |
| NYHA | ||||
| II | 73.4% (422) | 71.7% (412) | .509 | 0.04 |
| III-IV | 26.6% (153) | 28.3% (163) | .509 | –0.04 |
| LVEF, % | 28.36±6.88 | 28.36±6.87 | .997 | 0.00 |
| Grade III-IV mitral regurgitation | 15.1% (87) | 15.1% (87) | 1 | 0.00 |
| Idiopathic cardiomyopathy | 27.5% (158) | 25.6% (147) | .462 | 0.04 |
| NT-proBNP, ng/L | 1362 [528-4145] | 1550 [605-3905] | .909 | –0.01 |
| Hemoglobin, g/dL | 13.7±1.6 | 13.6±1.8 | .639 | 0.03 |
| eGFR, mL/min/1.73 m2 | 63.1±21.0 | 62.9±23.9 | .873 | 0.01 |
| Individual treatments | ||||
| ACEIs (or ARBs) | 89.6% (515) | 86.3% (496) | .086 | 0.11 |
| Beta-blockers | 70.4% (405) | 88.0% (506) | <.001 | –0.39 |
| Mineralocorticoid antagonist | 44.3% (255) | 63.1% (363) | <.001 | –0.38 |
| Automatic defibrillator | 1.6% (9) | 22.1% (127) | <.001 | –1.58 |
| Resynchronization therapy | 4.7% (27) | 8.5% (49) | .009 | –0.18 |
| Combined treatments | ||||
| Total (n) | 2.106±0.852 | 2.680±0.916 | <.001 | –0.67 |
| Categories | <.001 | |||
| 0 | 2.1% (12) | 1.0% (6) | 0.08 | |
| 1 | 20.9% (120) | 8.2% (47) | 0.31 | |
| 2 | 45.0% (259) | 29.2% (168) | 0.32 | |
| 3 | 29.0% (167) | 47.8% (275) | –0.41 | |
| 4 | 2.3% (13) | 10.8% (62) | –0.56 | |
| 5 | 0.7% (4) | 3.0% (17) | –0.26 | |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor antagonists; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT–proBNP, N-terminal pro–brain natriuretic peptide; NYHA, New York Heart Association.
Data are expressed as % (No.), mean±standard deviation, or median [interquartile range].
Matched populations in the propensity analysis. Function of cumulative incidence of mortality and causes of death in the MUSIC (M) and REDINSCOR I (R) registry populations, for the overall population and according to NYHA functional class. A: total mortality in the overall population; B: total mortality in NYHA II patients; C: total mortality in NYHA III-IV patients; D: causes of death in the overall population; E: causes of death in NYHA II patients; F: causes of death in NYHA III-IV patients. HF, heart failure; NYHA, New York Heart Association; SD, sudden death.
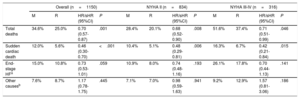
Analysis of regression and competing risks of death at 48 months in propensity score matched populations: REDINSCOR I vs MUSIC
| Overall (n=1150) | NYHA II (n=834) | NYHA III-IV (n=316) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | R | HR/sHR (95%CI) | P | M | R | HR/sHR (95%CI) | P | M | R | HR/sHR (95%CI) | P | |
| Total deaths | 34.6% | 25.0% | 0.70 (0.57-0.87) | .001 | 28.4% | 20.1% | 0.68 (0.52-0.90) | .008 | 51.6% | 37.4% | 0.71 (0.51-0.99) | .046 |
| Sudden cardiac death | 12.0% | 5.6% | 0.46 (0.30-0.70) | <.001 | 10.4% | 5.1% | 0.48 (0.29-0.81) | .006 | 16.3% | 6.7% | 0.42 (0.21-0.84) | .015 |
| End-stage HFa | 15.0% | 10.8% | 0.73 (0.53-1.01) | .059 | 10.9% | 8.0% | 0.74 (0.48-1.16) | .193 | 26.1% | 17.8% | 0.70 (0.44-1.13) | .141 |
| Other causesb | 7.6% | 8.7% | 1.17 (0.78-1.75) | .445 | 7.1% | 7.0% | 0.98 (0.59-1.63) | .941 | 9.2% | 12.9% | 1.57 (0.81-3.06) | .186 |
95%CI, 95% confidence interval; HF, heart failure; HR, hazard ratio; M, MUSIC; NYHA, New York Heart Association; R, REDINSCOR I; sHR, subhazard ratio.
The present study, based on clinical practice registries with well-described cohorts, has shown that the use of treatment based on therapies with proven benefits and recommended in clinical practice guidelines is linked to lower mortality, in particular the risk of sudden cardiac death, regardless of functional class.
Improvement in mortality rates according to causeThe analysis included 2351 outpatients with LVEF <40%, and both populations were followed up for 4 years. However, the 2 populations were not comparable in terms of risk characteristics or treatments received, as the more recent included patients in more advanced functional classes and with a clinical profile of greater risk, but also received better drug and device therapy. These findings may indicate a worsening profile of patients seen in participating units, as well as the gradual inclusion of evidence-based treatments. When the populations were considered as a whole, mortality was similar in both cohorts, and once functional class was considered, mortality and particularly sudden cardiac death were significantly lower in the more recent population despite the higher-risk profile of its patients. In this context, the propensity adjustment obtained 2 populations that were comparable in terms of risk factors but with similar differences in evidence-based treatments. This analysis confirmed a lower risk of death in the more recent population, mainly due to an approximate reduction of 50% in the risk of sudden cardiac death. This decrease was significant, both for patients in NYHA II and in the advanced classes III-IV. The magnitude of the decrease was similar in both functional classes, but the impact was stronger in NYHA II due to the greater relative contribution of sudden death to all-cause deaths in less advanced stages of the disease. Mortality due to end-stage HF was also lower, showing a clear trend; however, the difference was not statistically significant after the competing risks adjustment, indicating that, although there was an impact on HF progression, it was weaker or at least not as early as in sudden cardiac death. Last, as expected, other causes of death were similar in both populations, despite the therapeutic differences.
Decrease in sudden cardiac deathThe effect of treatment on mortality in patients with HF and reduced ejection fraction is based on individual randomized clinical trials of each drug. Shen et al.15 recently compared the cumulative incidence of sudden cardiac death in various clinical trials and observed a decrease in its incidence in the control groups as these groups incorporated evidence-based treatments over the years. The risk of sudden cardiac death dropped 44% between the various studies, a similar percentage to that observed in our analysis. However, our study has the added value that it reflects the impact on real-world clinical practice, an aspect investigated very little until now. Thorvaldsen et al.18 studied a cohort of 5908 patients with HF, LVEF<30%, and NYHA II-IV from 2003 to 2012, but no relationship was found between evidence-based treatments and an improvement in overall survival. However, this analysis did not consider the various causes of death or the interaction with functional class, which could affect the results observed. Indeed, there were no differences in overall survival in our study, a finding that only became evident after analyzing causes of death and functional classes separately, because the more recent population includes patients with more advanced stages of the disease. Therefore, our results apply the findings reported by Shen et al.15 to clinical practice patients, in whom improved treatment led to a lower risk of death, particularly sudden cardiac death, regardless of functional class.
Influence of changes in cause of deathBecause the changes had a greater impact on sudden cardiac death than on end-stage HF death, sudden cardiac death accounted for a lower percentage of all-cause mortality. Consequently, there have also been changes in the causes of death, as patients experience longer survival and finally die from end-stage HF. This could have repercussions for the ICD indication, in particular for patients with a lower risk of sudden death, such as those with no prior infarction. A recent clinical trial found that ICD indication based only on LVEF was not associated with a reduction in overall mortality in patients with no prior infarction, who had high rates of drug-based treatments recommended by clinical practice guidelines.19 The role of drug therapy is also observed in our analysis, as the decrease in sudden cardiac death persisted after excluding the population with devices. Recently, dual neprilysin and angiotensin-receptor inhibition has reduced the risk of death, particularly of sudden cardiac death in patients with few symptoms.8 This drug, currently included in clinical practice, will have further impact on mortality and the cause of death in upcoming years.
Real-world adherence to treatment guidelinesIn our analysis, after the propensity score adjustment, the only difference was in the treatments received by patients. In particular, the rates were higher with drugs that are harder to introduce and to manage, such as beta-blockers and mineralocorticoid antagonists, and were highest with ICD implantations, in the case of devices. These rates were higher in the most recent registry (2007-2010) than in the European registry from the early 2000s (EuroHeart Failure Survey9) and similar to the rates reported by other European registries immediately afterwards, such as the ESC Heart Failure Long-Term Registry (2011-2013)20,21 and QUALIFY (2013-2014).22 This slow implementation of evidence-based therapies has been reported in numerous registries, and the reasons are multifactorial, including a lack of physician adherence to guidelines due to clinical inertia, lack of training, and other difficulties, for instance, higher comorbidity of patients in clinical practice.23 Apart from individual treatments, evidence-based treatment combinations were also more common in the more recent population. This fact is relevant, as the predictor analysis showed that the use of 2 or more evidence-based treatments was associated with a lower independent risk of sudden cardiac death. This finding is consistent with recent studies showing that a greater combination of treatments, and at least 50% of recommended doses, is associated with fewer deaths and hospitalizations.22,24
LimitationsThis study has several limitations, including a lack of data on the dosages used and on the length of treatments, as well as its use of an observational cohort with differences between populations. The propensity analysis was used to obtain 2 groups of patients with clinical characteristics that were similar and thus comparable despite the distinct time periods, differing only in the treatment variable, with treatment possibly leading to residual differences in the matched samples apart from the study variable. Additionally, some effect could have resulted from other unmeasured variables, for instance, patient adherence, time from diagnosis, organizational improvements, or improvements in other treatments, such as coronary revascularization. Moreover, the participation of various sites in the 2 registries could have led to a selection bias derived from the patient profile seen at each site. The sites comprising the 2 study periods were not entirely the same, with only 6 of the 18 sites participating in both registries, making it impossible to consider the cluster effect of the sites for the propensity score estimate. Cause of death is often hard to determine, although this limitation was minimized by the use of a predefined independent committee and blind validation. Despite these limitations, the analyses support the findings and allow randomized trial results16 to be applied to clinical practice, considering the variability of real-world clinical practice.
CONCLUSIONSIn patients with HF and reduced ejection fraction, improved evidence-based medical treatment is associated with a decrease in deaths in clinical practice registries, mainly due to a significantly lower risk of sudden cardiac death regardless of functional class. These results confirm the need for strategies to encourage the inclusion of evidence-based treatments in clinical practice.
FUNDINGThis study was partially funded by the Carlos III Health Institute, Madrid, Spain (RD12/0042/0049) (PI14/01637; INT16/00172) and by FEDER funds (CIBER Cardiovascular; CB16/11/00385).
CONFLICTS OF INTERESTNone related to this study.
- –
Based on decreased mortality rates observed in clinical trials, treatments have been recommended for patients with HF and reduced LVEF.
- –
There is a paucity of data on actual use in clinical practice and on the effects on the various causes of death.
- –
In real-world clinical practice, the use of evidence-based therapies for patients with HF and reduced LVEF is associated with a lower risk of mortality.
- –
The improvement in survival was mainly due to the significantly reduced risk of sudden cardiac death regardless of functional class.
- –
Therefore, these therapies lead to greater survival and to changes in the cause of patient death.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2019.09.030