About 5% to 10% of myocardial infarction (MI) patients show no signs of obstructive disease (≥ 50%) in the main epicardial coronary arteries.1 This condition is known as MI with nonobstructive coronary arteries (MINOCA). From the clinical perspective, the presentation of MINOCA is similar to that of MI and even meets the fourth universal definition of myocardial infarction (2018).2 Given the implementation of increasingly accurate diagnostic methods, such as high-sensitivity troponin assays, the working diagnosis of this condition is more and more common. However, there is considerable heterogeneity among the causes to be considered in this setting. The most frequent etiologies are myocarditis, myocardial ischemia due to mechanisms other than atherosclerotic plaque rupture or erosion (eg, spontaneous coronary artery dissection and coronary vasospasm), and takotsubo syndrome.3 Another possible cause to consider in the working diagnosis of MINOCA is coronary microvascular dysfunction. This is caused by microvascular dysfunction or obliteration of the microvasculature in patients with coronary embolism or microvascular spasm, which are largely evaluated via invasive functional studies.3,4 Elucidation of the etiology is important due to the varying approaches and prognoses of these conditions.1 Indeed, the prognosis is particularly unfavorable if the cause is not identified; under these conditions, the entity is classified as MINOCA with no specific underlying etiology.5
In this regard, a major role in the etiological diagnosis of MINOCA is potentially played by cardiac magnetic resonance (CMR), which can be used to characterize the myocardium and detect contractility changes and other structural and functional defects.3,6 In addition, recent technological advances in CMR have permitted the quantitative measurement of myocardial perfusion and the detection of global perfusion deficits in coronary microvascular disease.7 A common characteristic of most of the potential causes of MINOCA is their transient nature or manifestation. Thus, essential factors are not only the use of appropriate diagnostic methods such as CMR that shed light on the final diagnosis, but also their timing, in order to improve their diagnostic yield.
In a recent article published in Revista Española de Cardiología, Juncà et al.8 aimed to establish the diagnostic yield of CMR, as well as its ideal timing, in patients with a working diagnosis of MINOCA. To do so, the authors studied a cohort of 207 consecutive patients (mean age, 50 years; 60% men) assessed using CMR after a working diagnosis of MINOCA in a Spanish publicly-funded tertiary health care center between 2009 and 2022. The data were retrospectively collected and the authors excluded patients presenting with acute heart failure, a nonsinusal rhythm, or any contraindication to CMR. The definition of MI was based on the 2018 expert consensus document2 and, to rule out significant obstructive disease in the coronary arteries, catheter coronary angiography or computed tomography coronary angiography was performed.
A final diagnosis after CMR was reached in 91% of patients: myocarditis in 45% of these patients, MI in 20%, takotsubo syndrome in 19%, and other cardiomyopathies in 7%. To elucidate the ideal CMR timing in this setting, the sample was divided into 2 groups: an early group and a late group. The time to CMR was defined as the number of days from hospital admission to CMR performance and was 5 [interquartile range, 4-6] days for the patients in the early group and 10 [8-12] days for those in the late group. Early CMR was associated with a better diagnostic yield vs late CMR (96% vs 86%). Although myocarditis was the most frequent diagnosis in both groups, it was more common in the early CMR patients (53% vs 35%). The authors concluded that CMR has a very high diagnostic yield in patients with a working diagnosis of MINOCA, particularly when the scan is performed in the first week after presentation.
These results are relevant for clinical practice. First, the findings reveal the value of CMR in the etiological diagnosis of MINOCA, given its good diagnostic yield, in line with previous evidence.5 Second, the data indicate that CMR should be performed in the first week, due to the even higher associated diagnostic yield. However, this second point should be interpreted with caution and deserves a more in-depth debate.
The findings to be evaluated in CMR in these patients included the presence of late gadolinium enhancement foci or changes in regional contractility, and edema and myocardial inflammation emerged as common denominators in most causes of MINOCA.6 Because this edematous or inflammatory response often shows a dynamic behavior, it is important to perform CMR in the optimal window to avoid missing pertinent information. However, the etiological possibilities are highly variable, and their behavior follows suit. For example, edema developing in the context of MI exhibits a bimodal behavior.9 The first wave of edema, which appears suddenly after reperfusion and dissipates about 24hours after, is directly related to the reperfusion process itself, whereas the second wave of edema, which gradually appears in the days after the MI and reaches a plateau between 4 and 7 days later, is largely due to myocardial tissue scarring processes.9,10 In the case of takotsubo syndrome, research indicates the presence of edema and contractility changes when CMR is performed at an early stage (2-4 days) but rapid normalization of these abnormalities in imaging studies performed during follow-up (1-4 months).11 Finally, in patients with myocarditis, CMR has demonstrated a good diagnostic yield in the detection of edema in patients studied in the first 2 weeks after symptom onset, and its diagnostic yield decreases from day 14.6,12
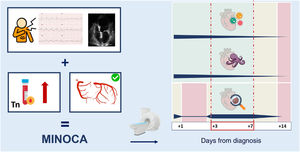
There are currently no clear recommendations on the ideal time to perform CMR in the context of MINOCA. Most guidelines and consensus documents indicate that the test should be performed during the hospital admission or in the first 2 weeks.3,13 Nonetheless, based on the results obtained by Juncà et al.8 and the literature,14 we can suggest an interval for CMR performance that runs from the third to the seventh day after symptom onset for the optimal diagnostic yield in patients with a working diagnosis of MINOCA (figure 1). The CMR protocol ideally should include myocardial perfusion and tissue characterization with T1 and T2 mapping, which exhibit higher sensitivity and specificity than other classic techniques for the detection of myocardial edema and which, accordingly, should further increase the diagnostic yield of CMR in this setting.6,15 All of the above depends on the availability and logistics of each center.
The left part of the figure summarizes the criteria for the working diagnosis of myocardial infarction with nonobstructive coronary arteries (MINOCA): a) symptoms of myocardial ischemia, electrocardiographic abnormalities compatible with ischemia, and/or changes in regional contractility on echocardiography; b) increase and/or decrease in the troponin level with at least 1 value above the 99th percentile; and c) absence of obstructive epicardial coronary artery disease ≥ 50%. Once the working diagnosis of MINOCA is made, the etiology must be investigated. Cardiovascular magnetic resonance is a test whose diagnostic yield depends on when it is performed. The right part of the figure shows a graphical representation of the times (in days since diagnosis) for a better diagnostic yield, depending on the underlying cause. The figure includes the most frequent causes of MINOCA: myocarditis, takotsubo syndrome, and myocardial infarction. Cardiovascular magnetic resonance should be performed between days 3 and 7. Tn, troponin. This figure has been designed using images obtained from Flaticon.com.
No funding was received for this article. C. Real receives funding from the “La Caixa” Foundation under project code LCF/PR/HR22/52320018. R. Fernández-Jiménez is a recipient of a Proyecto de Investigación en Salud grant with reference number PI22/01560 financed by the Instituto de Salud Carlos III (ISCIII) and cofunded by the European Union. The CNIC receives support from ISCIII, the Spanish Ministry of Science, Innovation, and Universities, and Fundación Pro CNIC and is a Severo Ochoa Center of Excellence (grant number CEX2020-001041-S, funded by MICIN/AEI/10.13039/501100011033).
CONFLICTS OF INTERESTThe authors declare that they have no conflicts of interest related to the content of this article.