Ranolazine is used as a complementary treatment for angina in symptomatic patients who are inadequately controlled with first-line antianginal therapies. Ranolazine inhibits sodium voltage-dependent channels, suggesting their possible involvement in the reperfusion process by preventing the sodium and calcium overload that occurs during ischemia. In this study, we characterized the effect of ranolazine on calcium homeostasis in isolated adult cardiac myocytes from rats subjected to a simulated ischemia and reperfusion protocol.
MethodsThe effects of ranolazine on changes in intracellular calcium concentration were evaluated at different times using field electrostimulation. The study of intracellular calcium was performed using microfluorimetry with the fluorescent indicator, Fura-2, and by confocal microscopy with the indicator, Fluo-3.
ResultsWe found that cardiomyocytes subjected to ischemia-reperfusion showed an increase in the diastolic calcium concentration and a decrease in the amplitude of intracellular calcium transients. The application of ranolazine during ischemia significantly improved intracellular calcium handling, preventing intracellular calcium overload, decreasing the diastolic calcium concentration, increasing the sarcoplasmic reticulum calcium load, and preserving the amplitude of the intracellular calcium transient, which was reflected by successful recovery in the process of excitation-contraction coupling during reperfusion. However, these effects of ranolazine did not occur when it was applied during reperfusion or when applied in both ischemia and reperfusion.
ConclusionsRanolazine shows beneficial effects in cardiomyocytes exposed to ischemia/reperfusion but only when applied during ischemia. This effect is achieved through its improvement of calcium handling during ischemia.
Keywords
Ischemic heart disease is the leading cause of death in the western world.1 The most devastating expression of this disease is ST-segment elevation myocardial infarction, which is due to acute coronary artery occlusion known to cause ischemic myocardial cell death. After ST-segment elevation myocardial infarction, rapid myocardial reperfusion by thrombolytic therapy or primary percutaneous coronary intervention is the most effective strategy to reduce myocardial infarct size and thus improve clinical outcome.2 Reperfusion therapy has a substantial impact on early mortality after ST-segment elevation myocardial infarction. However, a high percentage (20% to 30%) of patients develops adverse remodeling in our hospital.3 Early and effective reperfusion limits the extent of myocardial necrosis by reducing the incidence of left ventricular remodeling and dysfunction, but even with the best reperfusion therapy, paradoxically, a proportion of cardiomyocytes dies due to restoration of blood flow.4 This phenomenon is called ischemia/reperfusion (I/R) injury.5
Oxygen deprivation and calcium overload during cardiac ischemia and reactive oxygen species production during reperfusion cause cardiomyocyte death by necrosis and apoptosis.6 The major adverse changes that occur in the ischemic myocardium consist of an increase of intracellular Na+ concentration due to failure of the sarcolemma Na+/K+ pump in the absence of energy and acidification of cytosol by anaerobic glycolysis. The cell attempts to solve this intracellular Na+ increase through the Na+/Ca2+ exchanger (NCX) acting in the reverse mode, extruding Na+ and introducing Ca2+ inside the cells. This leads to an intracellular Ca2+ overload and a mishandling of Ca2+ by the cells.7
Ranolazine is a piperazine derivative with a novel mechanism of action that was first approved by the Food and Drug Administration in 2006 for the symptomatic treatment of patients with chronic angina. Due to its pharmacological properties, it is able to block the late Na+ current (INaL) in cardiomyocytes and steer the oxidation of fatty acids toward glucose oxidation, making oxygen use more efficient in the heart.8,9 However, the mechanism of action of ranolazine is not yet precisely known. The beneficial effects of ranolazine reside in its action of reducing the Na+ influx into myocardial cells through Na+ channels, which in pathologic situations fail during their inactivation or else they open again.10 Ranolazine has also been suggested to decrease calcium overload in myocardial cells during ischemia by blocking the INaL current.9 At therapeutic plasma concentrations (≤ 10-21 mmol/L), ranolazine selectively inhibits INaL, reduces intracellular accumulation of Na+ and subsequent Na+-induced Ca2+ overload, as well as mechanical, electrical, and metabolic abnormalities in ischemic or insufficient myocardium.11 However, at this concentration, ranolazine does not alter the peak of Na+ current responsible for step 0 of the action potential, the input current of Ca2+, or the activity of NCX and Na+/H+ exchanger.12
Nowadays, the use of ranolazine has been approved as an adjunctive therapy for symptomatic angina in patients who are inadequately controlled with first-line antianginal therapies.12 The development of a substance capable of inhibiting or reducing the deleterious effects of a pathological increase in intracellular Ca2+ concentration in cardiomyocytes during ischemia processes would be a hugely important clinical and therapeutic contribution. In this study, we hypothesized that ranolazine might have a novel action in reperfusion procedures, preventing the Na+ and Ca2+ overload that occurs in ischemic hearts and helping cells to improve Ca2+ handling at reperfusion.
METHODSAnimals were handled in accordance with the recommendations of the Royal Decree 53/2013 in agreement with Directive 2010/63/EU of the European Parliament. The study was approved by the local Ethics Committee on Human Research of the Virgen del Rocío University Hospital of Seville and the Animal Research Committee of the University of Seville.
Isolation of Ventricular MyocytesWe used adult male Wistar rats weighing approximately 250g to 350g, which were previously heparinized (4 IU/g intraperitoneally) and anesthetized by intraperitoneal administration of sodium thiopental (1mL/250g). The heart was quickly removed and mounted on a Langendorff perfusion system with a constant flow. Ventricular myocytes were isolated by perfusion using type II collagenase (251 IU/mL, Worthington Biochemical; Lakewood, New Jersey, United States).13 Cardiomyocytes were maintained in the Tyrode solution (mM): 140 NaCl, 4 KCl, 1.1 MgCl2, 10 HEPES, 10 glucose, 1.8 CaCl2 (pH 7.4), supplemented with 1.8mM CaCl2. All experiments were conducted on rod-shaped cells at room temperature (24°C to 26°C).
Intracellular Ca2+ Measurement With MicrofluorimetryIntracellular Ca2+ transients were recorded using the imaging system Incyt hight speed Im2 (Intracellular Imaging Inc.; Imsol, United Kingdom) in freshly isolated cardiomyocytes loaded with the fluorescence Ca2+ dye, Fura-2AM. During experiments, cells were continuously superfused with normal Tyrode (mM) or with simulated ischemia (mM): 140 NaCl, 3.6 KCl, 1.2 MgCl2, 1.8 CaCl2, 20 HEPES, 20 Lactate-Na and 2 NaCN (pH 6.22). To evoke intracellular Ca2+ transients, cardiomyocytes were field stimulated throughout the experiment at 0.5Hz using 2 parallel platinum electrodes, as previously described.14
Intracellular Ca2+ Measurement With Confocal MicroscopyConfocal microscopy experiments were performed in freshly isolated cardiomyocytes loaded with Fluo-3AM. Images were obtained with confocal microscopy (Leica TCS SP2 AOBS, objective W.I. 63×and N.A. 1.2) by scanning the cell with an Argon laser every 1.54ms. To evoke intracellular Ca2+ transients, the cardiomyocytes were field stimulated at 0.5Hz, as previously described.14 The sarcoplasmatic reticulum Ca2+ load was estimated by rapid caffeine application in Fluo-3-loaded cardiomyocytes using the Leica SP5, objective 40 × W.I., N.A. 1.2, scanning in the line scan mode at 700Hz, with a white light laser at 500nm. In both cases, emission was collected at > 510nm, as previously described.14,15
Treatment ProtocolsGroup 1: ischemia/reperfusion (I/R). After stabilization with control solution, cells were exposed to simulated ischemia by the perfusion of ischemic solution for 6minutes, followed by 10minutes of reperfusion with control solution.
Group 2: ranolazine applied at the onset of reperfusion (I/R+Ran). Cells were exposed to 6minutes of simulated ischemia and 10min of reperfusion with the control solution containing 10μM of ranolazine.
Group 3: ranolazine applied at ischemia and during reperfusion (I+Ran/R+Ran). Cells were exposed to 6minutes of simulated ischemia in the presence of 10μM of ranolazine followed by 10minutes of reperfusion with control solution containing 10μM of ranolazine.
Group 4: ranolazine applied only during ischemia (I+Ran/R). Cells were exposed to 6minutes of simulated ischemia with 10μM of ranolazine followed by 10minutes of reperfusion with control solution.
Using these protocols, 50% to 60% of cardiomyocytes subjected to I/R protocol showed a significant hypercontraction compared with control cells.
Data AnalysisGroup data are presented as mean ± (standard error of the mean). The single or paired Student's t test was used to determine the statistical significance of the data. The significance between multiple groups was evaluated using analysis of variance followed by the Tukey test. Results with a P-value < .05 were considered significant. Drugs were purchased from Sigma–Aldrich.
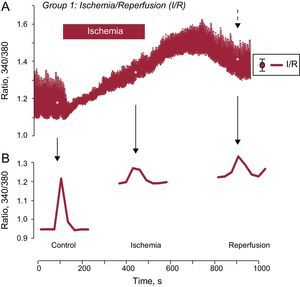
RESULTSIntracellular Ca2+ Changes During Ischemia-reperfusionChanges in intracellular Ca2+ were examined in cardiomyocytes subjected to I/R that were field stimulated at 0.5Hz. Ischemia induced a significant decrease in the amplitude of intracellular Ca2+ transients that was partially recovered after reperfusion (Figure 1A). This effect was accompanied by an increase in diastolic intracellular Ca2+ level during ischemia and during early reperfusion and was not restored at the end of reperfusion (Figure 1B). As depicted, intracellular Ca2+ baseline levels were stable in the control situation, but continued to increase during simulated ischemia, impeding the recovery of Ca2+ transient at reperfusion.
Effect of ischemia-reperfusion on intracellular Ca2+ transients. A: shows the mean trace of changes in intracellular Ca2+ transients from several isolated adult cardiomyocytes loaded with the fluorescent indicator, Fura-2AM, and subjected to a protocol of simulated I/R. The cells were stimulated at a frequency of 0.5Hz. B: shows a representative individual [Ca2+]i transient recorded in control solution, during ischemia, and after cell reperfusion. I/R: ischemia/reperfusion. The dashed arrow indicates the standard error of the mean, which is shown on the right in Figure A. *P < .05.
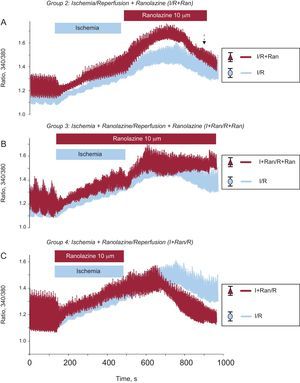
To check the effect of ranolazine on intracellular Ca2+ handling, we first sought to determine the appropriate timing of its application. To do this, isolated adult cardiomyocytes were subjected to a protocol of I/R in which the drug was applied in 3 stages: a) at the time of reperfusion; b) during I/R,and c) during ischemia only. First, we applied ranolazine (10 μM) at the onset of reperfusion as a treatment suitable for clinic applications during primary angioplasty and therefore cardiac cells were subjected to simulated ischemia for 6minutes and were then exposed to 10minutes of reperfusion with a control solution containing 1.8mM CaCl2 and 10μM ranolazine (Figure 2A). Using this protocol, we observed no improvement in the change of intracellular Ca2+. Moreover, the increase of diastolic intracellular Ca2+ caused by ischemia further increased with the application of the drug at the time of reperfusion.
Effect of ranolazine applied in different experimental groups. Traces show the time course of changes in the mean intracellular Ca2+ transients recorded in cardiomyocytes subjected to I/R (blue traces; n=59) and in cells treated with ranolazine applied at different times (red traces). A: ranolazine (10μM) was applied at the onset of reperfusion (n=16). B: ranolazine (10μM) was added during ischemia and reperfusion (n=24). C: ranolazine (10μM) was applied during ischemia only (n=31). I+Ran/R, ischemia + ranolazine/reperfusion; I+Ran/R+Ran, ischemia + ranolazine/reperfusion + ranolazine; I/R, ischemia/reperfusion; IR+Ran, ischemia-reperfusion + ranolazine. The dashed arrow indicates the standard error of the mean, shown on the right.
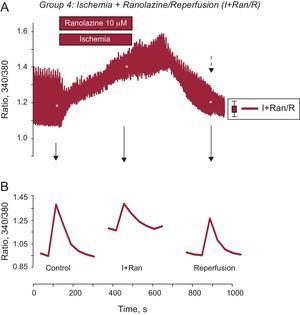
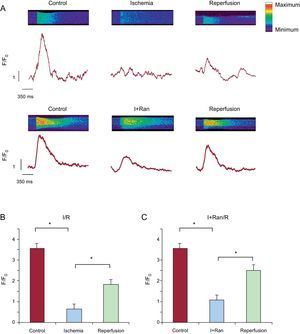
Next, to determine whether ranolazine had a greater protective effect when applied during I/R, cardiomyocytes were treated with a solution of simulated ischemia supplemented with 10μM ranolazine for 6minutes and were then reperfused with control solution also supplemented with ranolazine for 10minutes. As a result of this experiment, we observed a progressive and sustained increase in diastolic intracellular Ca2+ that did not improve at any time during reperfusion (Figure 2B). Finally, adult cardiomyocytes were subjected to 6minutes of simulated ischemia solution containing 10μM ranolazine, followed by 10min of reperfusion in the absence of the drug. The increase in diastolic intracellular Ca2+ generated during ischemia decreased significantly at reperfusion with the control solution (Figure 2C). After application of ranolazine during simulated ischemia only, the intracellular Ca2+ transient significantly improved at reperfusion in cardiac cells compared with the other others protocols (Figure 3). Indeed, the Ca2+ overload generated by ischemia was significantly reduced during reperfusion in this group. In addition, the intracellular Ca2+ transient amplitude recovered significantly during reperfusion without ranolazine (Figure 3B).
Ranolazine addition during ischemia restored the amplitude of intracellular [Ca2+] transients. A: traces show the mean for recording of intracellular Ca2+ transients in cardiomyocytes treated with ranolazine applied during ischemia (grey). B: representative recording of an individual intracellular Ca2+ transient showing significant recovery at reperfusion. The dashed arrow indicates the standard error of the mean, shown on the right. I+Ran, ischemia + ranolazine; I+Ran/R, ischemia + ranolazine/reperfusion. *P < .05.
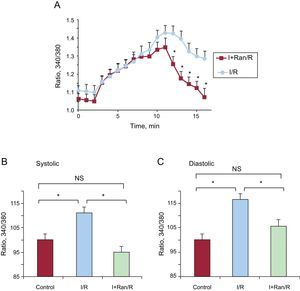
A detailed analysis of diastolic intracellular Ca2+ showed that it increased progressively, was higher in the I/R group, and did not recover initial values at reperfusion. Meanwhile, the addition of ranolazine to the ischemic solution produced a similar intracellular Ca2+ increase during ischemia, but in this case it was restored to preischemic values at reperfusion without ranolazine (Figure 4A). Treating the cells with ranolazine during ischemia restored the systolic increase of intracellular Ca2+ to levels similar to preischemic values (Figure 4B). Diastolic intracellular Ca2+ decreased during reperfusion in the I+Ran/R group (105.86%±3.07%) and was significantly lower than in the I/R group (116.65%±1.83%) (Figure 4C).
Ranolazine restored the diastolic intracellular Ca2+ transients at reperfusion after ischemia. A: graphic representation of changes in diastolic intracellular Ca2+ during the experiment in cardiomyocytes submitted to I/R and cardiomyocytes submitted to I+Ran/R. The concentration of diastolic Ca2+ recovered significantly in cells treated with ranolazine. B: the bar graph summarizes the amplitude of end systolic intracellular Ca2+ in control solution, I/R (n=59), and I+Ran/R (n=30). C: the bar graph summarizes the amplitude of end diastolic intracellular Ca2+, in control solution, I/R (n=59), and I+Ran/R (n=30). I+Ran/R, ischemia + ranolazine/reperfusion; I/R, ischemia/reperfusion; NS, not significant. *P < .05.
To gain greater insight into the cardiomyocyte contraction process, we repeated the experiments by confocal imaging using the line scan model.14 By scanning the long axis of the cardiomyocytes, the “rigor” of the cells can be followed as the contractures during diastole. First, we analyzed intracellular Ca2+ transients, which confirmed the results of Ca2+ measurements with microfluorimetry (Figure 5). We observed a marked recovery of intracellular [Ca2+] transients after submission of cardiomyocytes to the IR protocol, using ranolazine (10μM) during ischemia and removing it at the beginning of reperfusion (Figure 5A). The bar graph in Figure 5B indicates a significant decrease of the amplitudes of intracellular [Ca2+] transients that recovered partially during reperfusion. Meanwhile, Figure 5C shows a significant recovery in the amplitude of the intracellular [Ca2+] transients corresponding to the I+Ran/R protocol. Figure 5C also shows that the amplitude of intracellular [Ca2+] transients during ischemia was maintained in the presence of ranolazine but not in its absence.
Effect of ranolazine on intracellular Ca2+ transients measured using confocal microscopy. A: representative line scan images (upper panel) showing intracellular Ca2+ transients in cardiomyocytes from I/R and I+Ran/R stimulated at a frequency of 0.5Hz and the corresponding amplitude of these transients (lower panel). B and C: bar graphs showing summary data of the change in the amplitude of intracellular Ca2+ transients in experiment as in (A) (n=4-6). I+Ran, ischemia + ranolazine; I+Ran/R, ischemia + ranolazine/reperfusion; I/R, ischemia/reperfusion. * P < .05.
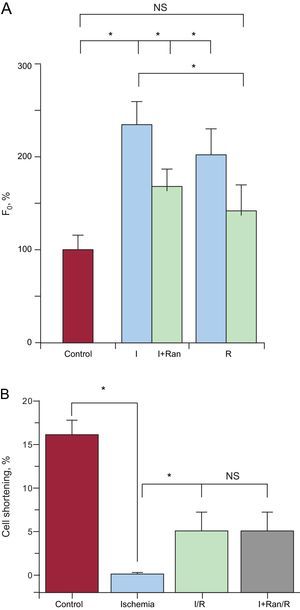
Furthermore, the pattern of diastolic intracellular [Ca2+] using confocal microscopy showed a significant increase in diastolic intracellular [Ca2+] during ischemia and a minimum recovery on reperfusion in cells subjected to I/R (Figure 6A). However, compared with the I+Ran/R protocol, the increase in diastolic intracellular [Ca2+] during ischemia and at reperfusion was lower than values in the I/R group. Finally, ranolazine did not improve cell shortening recovery in cells submitted to the I+Ran/R protocol compared with those subjected to I/R (Figure 6B).
Changes in diastolic intracellular Ca2+ in cells treated with ranolizine. A: bar graph comparing the amplitude of diastolic Ca2+ concentration recorded in control solution (red), ischemia, and reperfusion in the ischemia-reperfusion (blue, n=6) and in ischemia + ranolazine/reperfusion (green, n=4) using confocal microscopy. B: cell shortening in cardiomyocytes (n=10) from control solution (red), ischemia (blue), ischemia-reperfusion (green) and ischemia + ranolazine/reperfusion (grey). I+Ran, ischemia + ranolazine; I+Ran/R, ischemia + ranolazine/reperfusion; I/R, ischemia/reperfusion; NS, not significant. *P < .05.
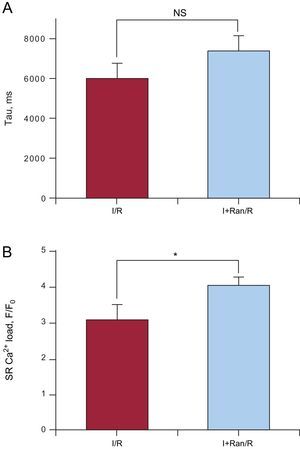
Because NCX is very important for Ca2+ homeostasis in adult cardiomyocyte, we tested its involvement in Ca2+ handling during I+Ran/R. For this purpose, we used I+Ran/R and, 3minutes after reperfusion, we applied 10mM of caffeine. In the presence of caffeine, we studied the tau of recovery to baseline Ca2+, which was mainly due to Ca2+ extrusion through the NCX. Ranolazine did not alter this parameter, suggesting that the NCX exchanger is not involved in the intracellular Ca2+ enhancement by ranolazine during ischemia (Figure 7A). However, the analysis of sarcoplasmic reticulum Ca2+ load, estimated by caffeine-evoked intracellular Ca2+ transients, indicates that it was higher in cells treated with ranolazine compared with untreated cells, indicating that ranolazine promoted sarcoplasmic reticulum load in cardiomyocytes (Figure 7B).
Effect of ranolazine effect on the Na+/Ca2+ exchanger and sarcoplasmic reticulum Ca2+ load. A: the bar graph shows the analysis of the decay in the intracellular Ca2+ transient (tau, milliseconds) induced by 10mM caffeine, which evaluates Na+/Ca2+ exchanger function. B: caffeine-evoked Ca2+ transients peak, which estimates sarcoplasmic reticulum Ca2+ load. I+Ran/R, ischemia + ranolazine/reperfusion; I/R, ischemia/reperfusion; NS, not significant; SR, sarcoplasmic reticulum. Data are from cells submitted to ischemia-reperfusion protocol and from cells treated with ranolazine in ischemia (ischemia + ranolazine/reperfusion). Summary data are from 37 cells. *Statistical significance.
Since the early 1950s, intensive research has been conducted in the field of myocardial protection, the main objective being to characterize the cellular and molecular mechanisms involved in myocardial protection after the myocardium has undergone a process of I/R.15 First, in a previous study,14 we confirmed the feasibility of our experimental I/R model for isolated cardiomyocytes, in which we were able to analyze in vivo structural and intracellular Ca2+ changes that occur in the process of I/R.14 Next, in the present study, we provide new evidence that ranolazine, which is widely used for antianginal therapies,16 can protect cardiomyocytes against I/R injury through regulation of intracellular Ca2+ handling independently of its effect on INaL. Using 2 different approaches to analyze intracellular Ca2+ changes, we studied changes in intracellular Ca2+ in cardiomyocytes subjected to ischemia and/or reperfusion and the role of ranolazine, which is able to reduce the Ca2+ overload generated in I/R processes. In this study, we confirm that application of ranolazine during myocardial ischemia significantly restores the amplitude of intracellular Ca2+ transients in reperfusion. Moreover, we not only noticed improvement in the amplitude of the transients but also observed that diastolic intracellular Ca2+, which remained elevated throughout ischemia, returned to baseline levels after treatment with ranolazine during ischemia. These data are consistent with studies conducted by Hwang et al,17 in Langendorff-perfused rat hearts, in which ranolazine was applied prior to ischemia. These authors observed that intracellular Ca2+ transients, abolished by the interruption of left ventricular function after a certain period of ischemia, were rapidly restored during postischemic reperfusion with ranolazine.17 However, in this study, residual intracellular Ca2+ remained significantly high compared with baseline values prior to ischemia induction.17 Furthermore, in this study, the analysis of caffeine-evoked intracellular Ca2+ transients suggested that ranolazine increased the efficiency of the sarcoplasmic reticulum Ca2+ load, which is essential to preserve efficient excitation-contraction coupling in cardiomyocytes after an ischemic period. Moreover, the analysis of the decay time constant of the intracellular Ca2+ transient (tau) showed that ranolazine treatment during ischemia did not enhance intracellular Ca2+ extrusion via the NCX. Thus, NCX seemed not to be implicated in the diastolic intracellular Ca2+ increase in reperfusion when cells were treated with ranolazine during ischemia. Therefore, alternative mechanisms must be involved in this effect of ranolazine on intracellular Ca2+, which are worth investigating.
Most previous studies have applied ranolazine prior to ischemia.17,18 Here, we applied the drug once ischemia had been started and/or at reperfusion. We aimed to reproduce what might happen in the clinical scenario when drugs should be administered at the onset of reperfusion. Significant beneficial effects on intracellular Ca2+ regulation were only observed only ranolazine was applied during ischemia, which might limit its use for clinical application in patients with ST-segment elevation myocardial infarction. However, we believe that it is still important to understand these effects on the Ca2+ homeostasis of cardiomyocytes in I/R.
In recent decades, there has been an explosion of information regarding changes in contractility following I/R myocardial syndrome, as in the case of processes known as “stunned myocardium” (stunning) and hibernating heart.19,20 Ca2+ plays a central role in the regulation of contraction and heart rate and has been associated with prevailing heart disease, directly or indirectly, and to changes in the behavior of intracellular Ca2+.21–23 The intracellular Ca2+ overload causes an increase in diastolic interaction of the myofilaments actin/myosin and increased left ventricular diastolic pressure (eg, “stiffness”, inability to relax normally). This diastolic disorder occurs due to prolongation of action potentials and slowing of biochemical pumps, which are necessary for the reuptake of intracellular calcium.24,25 As a result, contractile myocardial work, oxygen consumption, and the compression of the vascular space during diastole may be abnormally high. The compression of the vascular space leads to a reduction of myocardial blood flow that decreases oxygen supply, especially in the subendocardial region of the left ventricle, while increasing the demand for oxygen to support contractile work. This pattern of cause and effect has the characteristics of a deleterious positive “feedback” system, in which ischemia generates further ischemia.26,27 With the experiments performed in our research, we demonstrated that intracellular diastolic intracellular Ca2+ recovered significantly in adult rat cardiomyocytes subjected to a protocol of I/R when we applied ranolazine during ischemia. These data are in line with the results of other studies,18,28 in which the effect of ranolazine occurred with the inhibition of the INaL using a model of a full heart. Ranolazine reduced the overload of intracellular Ca2+, returning postischemic diastolic intracellular Ca2+ values close to baseline preischemic levels. Sossalla et al29 studied the potential effect of ranolazine on diastolic intracellular Na+ and Ca2+ overload and its ability to improve diastolic function in the muscle fibers of ventricles of human hearts from patients with end-stage heart failure undergoing heart transplantation.29 Furthermore, to investigate the beneficial effect of ranolazine on diastolic pressure, they used Anemonia sulcata toxin II (40nM) to increase intracellular Na+ concentration in rabbit ventricular myocytes. In the presence of ranolazine, INaL current, as well as diastolic intracellular Na+ and intracellular Ca2+, decreased in all pacing rates. In addition, ranolazine significantly accelerated the decline of the Ca2+ transient, which was initially slowed by A. sulcata toxin II.30
CONCLUSIONSIn conclusion, our results clearly show that, when administered during ischemia, ranolazine protects isolated adult rat cardiomyocytes from damage by I/R, improving the excitation-contraction coupling process and decreasing Ca2+ overload in reperfusion, which is of major interest to preserve heart contractility from I/R injuries.
FUNDINGThis study was supported by the Spanish Ministry of Science and Innovation (BFU2013-45564-C2-2); Instituto de Salud Carlos III (PI12/00941) and RIC (Red de Investigación Cardiovascular) (RD12/0042/0041; RD12/0042/0030); by The Andalusia Government (PI-0108-2012; P10-CVI-6095), and by the French ANR (Agence Nationale de la Recherche) (ANR-13-BSV1-0023-01).
CONFLICTS OF INTERESTNone declared.
We thank Florence Lefevbre for her help with cell isolation.

![Effect of ischemia-reperfusion on intracellular Ca2+ transients. A: shows the mean trace of changes in intracellular Ca2+ transients from several isolated adult cardiomyocytes loaded with the fluorescent indicator, Fura-2AM, and subjected to a protocol of simulated I/R. The cells were stimulated at a frequency of 0.5Hz. B: shows a representative individual [Ca2+]i transient recorded in control solution, during ischemia, and after cell reperfusion. I/R: ischemia/reperfusion. The dashed arrow indicates the standard error of the mean, which is shown on the right in Figure A. *P < .05. Effect of ischemia-reperfusion on intracellular Ca2+ transients. A: shows the mean trace of changes in intracellular Ca2+ transients from several isolated adult cardiomyocytes loaded with the fluorescent indicator, Fura-2AM, and subjected to a protocol of simulated I/R. The cells were stimulated at a frequency of 0.5Hz. B: shows a representative individual [Ca2+]i transient recorded in control solution, during ischemia, and after cell reperfusion. I/R: ischemia/reperfusion. The dashed arrow indicates the standard error of the mean, which is shown on the right in Figure A. *P < .05.](https://static.elsevier.es/multimedia/18855857/0000006900000001/v1_201601041128/S1885585715001796/v1_201601041128/en/main.assets/thumbnail/gr1.jpeg?xkr=eyJpdiI6IjFXeGxRNW5BK25YRUppcU14UExNTEE9PSIsInZhbHVlIjoiei9vNSttbWlZbEpuVUxKUXVnOHhhMTdFNzJiQWpRT2dOS1RLYmp1djVkdz0iLCJtYWMiOiI0MzU2MDc1Yzc3ZjQ4OGNiZWY4YjNlMDQ4OWE5NGM3MDFmYTA2OTIwOTNmZmZkYTM0Nzc5MGM3ZTgzY2ZlYjIzIiwidGFnIjoiIn0=)

![Ranolazine addition during ischemia restored the amplitude of intracellular [Ca2+] transients. A: traces show the mean for recording of intracellular Ca2+ transients in cardiomyocytes treated with ranolazine applied during ischemia (grey). B: representative recording of an individual intracellular Ca2+ transient showing significant recovery at reperfusion. The dashed arrow indicates the standard error of the mean, shown on the right. I+Ran, ischemia + ranolazine; I+Ran/R, ischemia + ranolazine/reperfusion. *P < .05. Ranolazine addition during ischemia restored the amplitude of intracellular [Ca2+] transients. A: traces show the mean for recording of intracellular Ca2+ transients in cardiomyocytes treated with ranolazine applied during ischemia (grey). B: representative recording of an individual intracellular Ca2+ transient showing significant recovery at reperfusion. The dashed arrow indicates the standard error of the mean, shown on the right. I+Ran, ischemia + ranolazine; I+Ran/R, ischemia + ranolazine/reperfusion. *P < .05.](https://static.elsevier.es/multimedia/18855857/0000006900000001/v1_201601041128/S1885585715001796/v1_201601041128/en/main.assets/thumbnail/gr3.jpeg?xkr=eyJpdiI6IlA5VDVDNElZaTVST0tQSGIxREJBRWc9PSIsInZhbHVlIjoiTWU3ZHM2M3FlcTB2K082TzY5d0RiT3kwWVMxMDN1VG5XeFJ6Zy9pbFlJQT0iLCJtYWMiOiIxNGYwMjNmNjdiMDk3NjBkNjMzZDM4YmJiNzZlZDhmN2VjMjc1YTI3MzI5YjU3MzdjZjE2M2MxNzZiMDkzYjVmIiwidGFnIjoiIn0=)



