To the Editor:
Sarcoidosis is a systemic disease of unknown etiology affecting the heart in 5%-10% of clinically studied cases. In autopsy studies, the incidence can be as high as 76%.1,2
It can progress rapidly and in such a way that sudden death may be the first symptom.3 Cardiac sarcoidosis is diagnosed following the criteria set out by the Japanese Ministry of Health, which include hemodynamic and electrocardiographic alterations, imaging techniques, and even myocardial biopsy.4 Early treatment improves the patient's prognosis.5 Among the imaging techniques, nuclear magnetic resonance imaging (MRI) with late enhancement through administering gadolinium has been a significant advance in confirming the diagnosis and evaluating the prognosis.6,7
We present the case of a 70-year old woman with chest pain, vasovagal syncope about 60 minute long and tinnitus in both ears.
One year earlier, due to feeling generally unwell, she underwent a radiological study in which interstitial lesions were found in both lungs; these were studied by a transbronchial biopsy, which uncovered the presence of noncaseating granulomas typical of sarcoidosis. Other causes of granulomatous lung disease were ruled out.
Physical examination showed normal blood pressure and an arrhythmic arterial pulse of 94 beats/min. The laboratory results showed the following: calcium, 11.1 mg/dL; angiotensin convertase, 40.3 U/dL; uric acid, 8.1 mg/dL; and creatinine, 1.7 mg/dL. Ergometric and scintigraphic studies of the myocardium at rest and following a stress test with 99mTc showed a paradoxical perfusion defect in the inner layer of the myocardium. The 24 h Holter showed infrequent monotopic ventricular extrasystoles, and the echocardiogram showed concentric hypertrophy of the left ventricle with relaxation abnormalities, but with preserved systolic function and moderate mitral regurgitation.
The MRI study showed ganglionic, pulmonary and cardiac alterations (Figures 1 and 2) and the coronary angiography revealed atherosclerotic plaques without hemodynamic significance in both coronary arteries.
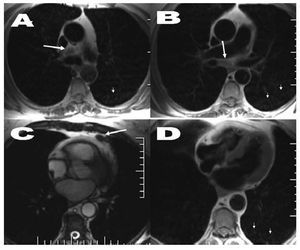
Figure 1. Axial T1 spin-echo sequence (A, B, D) and cine gradient-echo imaging (C). Images of mediastinal adenopathies of £1 cm in the following spaces or regions: inferior pretracheal-retrocaval (A), infracarinal (B), and left paracardial (C) (large arrows). Additionally, a pulmonary interstitial parenchymatous disorder can be seen which is micronodular, bilateral and vaguely defined (small arrows).
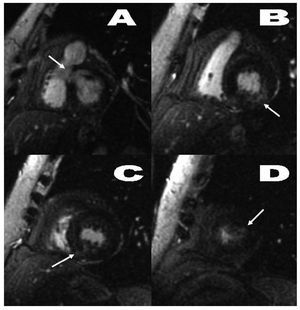
Figure 2. Gradient echo sequence with late contrast enhancement, short axis orientation. Patchy, nodular, poorly defined increase in intramyocardial signal intensity on the lower basal margin of the septum (A), posterior lateral side of the left ventricle (B), posterior medio-apical angle (C), and small focus on the apical lateral side (D) (arrows).
The diagnosis of cardiac sarcoidosis in patients with a previous diagnosis of sarcoidosis in other organs is confirmed by other means than with MRI. However, MRI enables viewing of the sarcoid lesions and monitoring the response to therapy, even in patients without cardiological symptoms.5 Using MRI, researchers have described mediastinal adenopathies, infiltration of the ventricular walls, late enhancing lesions with a nodular, patchy appearance indicating inflammation and myocardial fibrosis, and regional myocardial dysfunction.6-8
Our case, which meets the diagnostic criteria for cardiac sarcoidosis, enables illustrating the morphology of cardiac lesions using MRI. These lesions affect the basal septum in particular, as well as other parts of the myocardium without regional correspondence to the anatomic distribution of the coronary circulation. They appear scattered, patchy and nodular, at times with poorly defined borders, as other authors have described.6,7 The usefulness of MRI suggests the need to revise the diagnostic criteria for cardiac sarcoidosis that have been in use until now.