Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) is a metabolic regulator induced during ischemia that prevents cardiac remodeling in animal models. The activity of PGC-1α can be estimated in patients with ST-segment elevation acute myocardial infarction. The aim of the present study was to evaluate the value of blood PGC-1α levels in predicting the extent of necrosis and ventricular remodeling after infarction.
MethodsIn this prospective study of 31 patients with a first myocardial infarction in an anterior location and successful reperfusion, PGC-1α expression in peripheral blood on admission and at 72hours was correlated with myocardial injury, ventricular volume, and systolic function at 6 months. Edema and myocardial necrosis were estimated using cardiac magnetic resonance imaging during the first week. At 6 months, infarct size and ventricular remodeling, defined as an increase > 10% of the left ventricular end-diastolic volume, was evaluated by follow-up magnetic resonance imaging. Myocardial salvage was defined as the difference between the edema and necrosis areas.
ResultsGreater myocardial salvage was seen in patients with detectable PGC-1α levels at admission (mean [standard deviation (SD)], 18.3% [5.3%] vs 4.5% [3.9%]; P = .04). Induction of PGC–1α at 72hours correlated with greater ventricular remodeling (change in left ventricular end-diastolic volume at 6 months, 29.7% [11.2%] vs 1.2% [5.8%]; P = .04).
ConclusionsBaseline PGC–1α expression and an attenuated systemic response after acute myocardial infarction are associated with greater myocardial salvage and predict less ventricular remodeling.
Keywords
ST-segment elevation acute myocardial infarction (STEMI) is one of the main causes of death from cardiovascular disease.1 Survival and quality of life in patients with STEMI have recently been improved by advances in reperfusion strategies that reduce ischemia time. Nonetheless, many patients still have extensive myocardial necrosis that impacts their clinical outcome.2 Numerous studies have shown an acceleration of myocardial cell death associated with ischemia during the restoration of coronary blood flow, in a phenomenon known as reperfusion injury.3
Research into cardioprotective therapies that protect against ischemia-reperfusion (IR) injury thus provides an opportunity to reduce cell necrosis and consequently improve patient prognosis. However, many aspects of IR injury remain unknown.4–6 Various studies have evaluated the effect of defense mechanisms against oxidative stress in myocardial infarction models, such as the use of antioxidants7 or regulation of mitochondrial enzymes.8 Nonetheless, the existence of a coordinated mechanism regulating the mitochondrial protection system in tissue with high metabolic rates such as the heart remains unresolved.
The transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) acts as a master regulator of genes involved in oxidative metabolism and mitochondrial biogenesis and plays a fundamental role in the metabolic control of cardiac muscle and cardiomyocyte maturation/differentiation.9
Reduced myocardial expression of PGC-1α has been seen in animal models of cardiac ischemia after coronary artery ligation,10 and use of drug agonists of peroxisome proliferator-activated receptors (PPARs)11 that preserve PGC-1α expression can reduce infarct size and myocardial cell apoptosis and help to preserve ventricular function.
Mitochondrial homeostasis is key to myocardial resistance to ischemic and oxidative stress conditions. While genetic deletion of PGC-1α removes the ability of the myocardium to adapt to overload stimuli,12 overexpression of PGC-1α disproportionately increases the number of mitochondria, altering cardiomyocyte sarcomere structure and triggering contractile dysfunction.13 Thus, PGC-1α induction in response to cell damage from oxidative stress should be transient and proportional.14
Assessment of PGC-1α concentration after STEMI could help to evaluate myocardial recovery, with analysis of both the baseline values of the molecule and its induction following hypoxia, allowing estimation of cardiac cell resistance to ischemia.
Our research group has recently described the possibility of detecting PGC-1α induction in patients with STEMI from its level of expression in lymphocytes derived from peripheral blood samples.15 Patients with greater induction of PGC-1α had larger infarcts and more frequent left ventricular dysfunction during follow-up. This negative response could be partly explained by PGC-1α–mediated overexpression of adenine nucleotide translocase 1 (ANT-1), a component of the mitochondrial permeability transition pore (mtPTP) complex regulating myocardial cell apoptosis in ischemic conditions.16
To characterize the degree of PGC-1α expression in response to myocardial oxidative stress triggered by IR, the aim of the present study was to determine if the baseline values of the molecule and its induction after STEMI are related to necrosis extent, myocardial area at risk, and ventricular function, in order to define a cardioprotective activation profile.
METHODSSampleThe present study prospectively enrolled 31 patients attending our hospital who were diagnosed with STEMI in an anterior location and who underwent reperfusion from June 2009 to May 2011. Only patients with a first STEMI were included, with an anterior infarction (causative lesion in the proximal or middle segment of the anterior descending coronary artery) and reperfusion therapy by primary or rescue angioplasty. Following the clinical practice guidelines of the European Society of Cardiology,17 STEMI was diagnosed in patients with ischemic symptoms, persistent ST-segment elevation on electrocardiogram, and the characteristic increase in myocardial necrosis biomarkers. Patients were initially verbally informed of their participation in the study, later signing the corresponding informed consent forms approved by the Clinical Research Ethics Committee of the hospital. The exclusion criteria and data obtained during the study are detailed in the supplementary material.
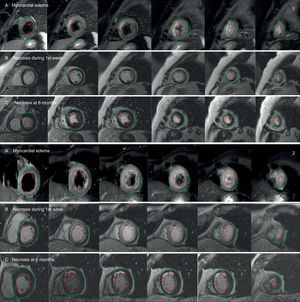
Cardiac Magnetic Resonance ImagingTwo cardiac magnetic resonance imaging scans were performed during follow-up. The first scan was performed between the third and fifth day after the infarction to evaluate the percentage of myocardial salvage (MS), defined as the difference between the myocardial edema in the acute phase of the infarction and the cardiac necrosis estimated by late gadolinium enhancement. The second follow-up scan was performed at 6 months to evaluate scar size. In addition, comparative analysis between the area of the myocardial edema in the initial scan and the necrotic area at 6 months allowed estimation of MS at the long-term follow-up (Figure 1).
Comparison of cardiac magnetic resonance imaging scans between 2 paradigmatic patients according to PGC-1α induction following myocardial infarction (1: patient with induction; 2: patient without induction): area at risk estimated by myocardial edema in T2-weighted Short Tau Inversion Recovery (T2-STIR) sequences (A), and area of necrosis estimated by late gadolinium enhancement during the first week (B) and at 6 months (C). The areas of necrosis and edema (maroon-colored lines) were calculated with respect to the total myocardial mass by manually tracing the epicardial (green line) and endocardial (red line) outlines.
The methodology of the cardiac magnetic resonance imaging scans are detailed in the supplementary material.
Preparation of Peripheral Blood Samples and Molecular Biology AnalysisPeripheral blood samples (6mL) were obtained using Vacutainer™ tubes containing EDTA on admission and at 72hours. Mononuclear cells were isolated using Ficoll-Hypaque (Ficoll-Paque™; Miltenyi Biotec) density gradient centrifugation. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was used to determine the relative PGC-1α RNA expression and its gene targets, cytochrome C, manganese superoxide dismutase (MnSOD), glutathione peroxidase (GPx), and ANT-1, using specific primers.18
Details of the RNA extraction and protein analysis are provided in the supplementary material.
For analysis of the results, the sample was divided into 2 groups as follows: (1) based on whether baseline PGC-1α expression was detectable in the first admission measurement; and (2) based on PGC-1α induction following the infarction, taking into account the RNA expression levels in the samples on admission and at 72hours. Induction of PGC-1α and ANT-1 was defined as a more than a 2-fold increase in qRT-PCR–determined expression of these 2 substances between the first and second samples.
Statistical AnalysisThe normality of the distribution of quantitative variables was determined using a Kolmogorov-Smirnov test. All continuous and normally distributed variables are expressed as mean (standard deviation), whereas categorical variables are expressed as number (percentage). Comparison of continuous variables between groups was performed using a Student t test for independent samples and a Mann-Whitney test for variables without normal distribution. Noncontinuous variables were compared using a chi-square test and Fisher's exact test when appropriate. The percentages of MS and ventricular remodeling (VR) were measured in each of the groups, and the means were also compared using a Student t test for independent samples. Analysis of long-term changes in the size of the necrotic zone and the MS was performed using a Student t test for paired samples. Error bar charts show the percentage change in the different subgroups. Statistical analysis was performed using SPSS for Windows version 17.0 (SPSS Inc; Chicago, Illinois, United States). P<.05 was considered statistically significant.
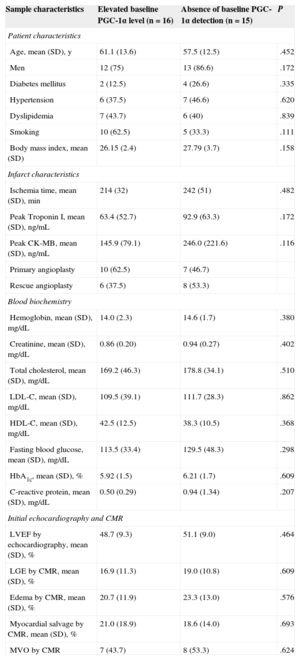
RESULTSBaseline Expression of PGC-1αBy dividing the sample based on the baseline PGC-1α expression in the admission blood samples, 2 clearly differentiated groups were established: patients with constitutive PGC-1α expression and patients without detectable baseline expression. The absence of initial peripheral detection of PGC-1α tended to be more frequent in diabetic patients and correlated positively but nonsignificantly with higher levels of C-reactive protein. No differences were found between the groups in cardiovascular risk factors and infarction characteristics (Table 1).
Sample Characteristics According to Baseline PGC-1α Expression: Cardiovascular Risk Factors, Infarct Characteristics, and General Biochemistry
| Sample characteristics | Elevated baseline PGC-1α level (n=16) | Absence of baseline PGC-1α detection (n=15) | P |
|---|---|---|---|
| Patient characteristics | |||
| Age, mean (SD), y | 61.1 (13.6) | 57.5 (12.5) | .452 |
| Men | 12 (75) | 13 (86.6) | .172 |
| Diabetes mellitus | 2 (12.5) | 4 (26.6) | .335 |
| Hypertension | 6 (37.5) | 7 (46.6) | .620 |
| Dyslipidemia | 7 (43.7) | 6 (40) | .839 |
| Smoking | 10 (62.5) | 5 (33.3) | .111 |
| Body mass index, mean (SD) | 26.15 (2.4) | 27.79 (3.7) | .158 |
| Infarct characteristics | |||
| Ischemia time, mean (SD), min | 214 (32) | 242 (51) | .482 |
| Peak Troponin I, mean (SD), ng/mL | 63.4 (52.7) | 92.9 (63.3) | .172 |
| Peak CK-MB, mean (SD), ng/mL | 145.9 (79.1) | 246.0 (221.6) | .116 |
| Primary angioplasty | 10 (62.5) | 7 (46.7) | |
| Rescue angioplasty | 6 (37.5) | 8 (53.3) | |
| Blood biochemistry | |||
| Hemoglobin, mean (SD), mg/dL | 14.0 (2.3) | 14.6 (1.7) | .380 |
| Creatinine, mean (SD), mg/dL | 0.86 (0.20) | 0.94 (0.27) | .402 |
| Total cholesterol, mean (SD), mg/dL | 169.2 (46.3) | 178.8 (34.1) | .510 |
| LDL-C, mean (SD), mg/dL | 109.5 (39.1) | 111.7 (28.3) | .862 |
| HDL-C, mean (SD), mg/dL | 42.5 (12.5) | 38.3 (10.5) | .368 |
| Fasting blood glucose, mean (SD), mg/dL | 113.5 (33.4) | 129.5 (48.3) | .298 |
| HbA1c, mean (SD), % | 5.92 (1.5) | 6.21 (1.7) | .609 |
| C-reactive protein, mean (SD), mg/dL | 0.50 (0.29) | 0.94 (1.34) | .207 |
| Initial echocardiography and CMR | |||
| LVEF by echocardiography, mean (SD), % | 48.7 (9.3) | 51.1 (9.0) | .464 |
| LGE by CMR, mean (SD), % | 16.9 (11.3) | 19.0 (10.8) | .609 |
| Edema by CMR, mean (SD), % | 20.7 (11.9) | 23.3 (13.0) | .576 |
| Myocardial salvage by CMR, mean (SD), % | 21.0 (18.9) | 18.6 (14.0) | .693 |
| MVO by CMR | 7 (43.7) | 8 (53.3) | .624 |
CK-MB, creatine kinase-MB fraction; CMR, cardiac magnetic resonance imaging; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MVO, microvascular obstruction; SD, standard deviation.
Statistical significance, P<.05. Data are expressed as No. (%) or mean (standard deviation).
To evaluate the role of baseline PGC-1α levels in postinfarction myocardial recovery, long-term MS was compared between the 2 groups. Although there were no differences in the MS in the initial examination, patients with higher PGC-1α expression at admission showed a higher MS index in the follow-up study (percentage of MS at 6 months, 39.3% [SD,4.5%] vs 23.2% [SD,5.6%]; P=.033) (Figure 2).
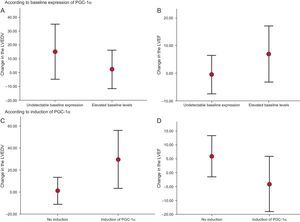
Moreover, ventricular volume analysis revealed a tendency toward greater VR in those patients without detectable baseline PGC-1α expression (percentage change in the left ventricular end-diastolic volume, 15.2% [SD,9.2%] vs 2.3% [SD,6.5%]; P=.26] (Figure 3A), whereas those patients with higher baseline PGC-1α levels had nonsignificantly improved systolic function at 6 months (percentage change in the left ventricular ejection fraction, 6.9% [SD,4.7%] vs –0.5% [SD,3.2%]; P=.20) (Figure 3B).
Change in ventricular volume and systolic function at 6 months (A and B). Change according to the baseline PGC-1α expression (C and D). Change according to PGC-1α induction after ST-segment elevation acute myocardial infarction. LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction.
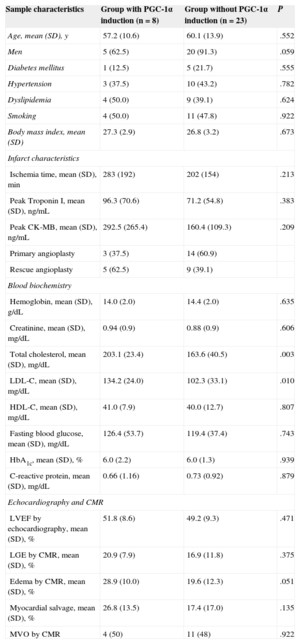
To study the functional impact of PGC-1α induction following STEMI, the sample was divided into 2 groups based on the presence or absence of PGC-1α, taking into account the degree of expression of PGC-1α in the blood samples at admission and at 72hours. Induction of PGC-1α was more frequently detected for large infarcts (necrotic and edema areas estimated by cardiac magnetic resonance imaging: late gadolinium enhancement, 20.99% vs 16.91%; P=.37; myocardial edema, 28.91% vs 19.59%; P=.051) and with nonsignificantly higher peak enzyme levels (peak troponin I level, 96.3 vs 71.2 ng/dL; P=.38; CK-MB, 292.5 vs 160.4 ng/dL; P=.20). Moreover, PGC-1α induction positively correlated with concentrations of total cholesterol and low-density lipoprotein cholesterol, but no other significant differences between the groups were found for cardiovascular risk factors and infarction characteristics (Table 2).
Sample Characteristics According to PGC-1α Induction Following Myocardial Infarction: Cardiovascular Risk Factors, Infarct Characteristics, and General Biochemistry
| Sample characteristics | Group with PGC-1α induction (n=8) | Group without PGC-1α induction (n=23) | P |
|---|---|---|---|
| Age, mean (SD), y | 57.2 (10.6) | 60.1 (13.9) | .552 |
| Men | 5 (62.5) | 20 (91.3) | .059 |
| Diabetes mellitus | 1 (12.5) | 5 (21.7) | .555 |
| Hypertension | 3 (37.5) | 10 (43.2) | .782 |
| Dyslipidemia | 4 (50.0) | 9 (39.1) | .624 |
| Smoking | 4 (50.0) | 11 (47.8) | .922 |
| Body mass index, mean (SD) | 27.3 (2.9) | 26.8 (3.2) | .673 |
| Infarct characteristics | |||
| Ischemia time, mean (SD), min | 283 (192) | 202 (154) | .213 |
| Peak Troponin I, mean (SD), ng/mL | 96.3 (70.6) | 71.2 (54.8) | .383 |
| Peak CK-MB, mean (SD), ng/mL | 292.5 (265.4) | 160.4 (109.3) | .209 |
| Primary angioplasty | 3 (37.5) | 14 (60.9) | |
| Rescue angioplasty | 5 (62.5) | 9 (39.1) | |
| Blood biochemistry | |||
| Hemoglobin, mean (SD), g/dL | 14.0 (2.0) | 14.4 (2.0) | .635 |
| Creatinine, mean (SD), mg/dL | 0.94 (0.9) | 0.88 (0.9) | .606 |
| Total cholesterol, mean (SD), mg/dL | 203.1 (23.4) | 163.6 (40.5) | .003 |
| LDL-C, mean (SD), mg/dL | 134.2 (24.0) | 102.3 (33.1) | .010 |
| HDL-C, mean (SD), mg/dL | 41.0 (7.9) | 40.0 (12.7) | .807 |
| Fasting blood glucose, mean (SD), mg/dL | 126.4 (53.7) | 119.4 (37.4) | .743 |
| HbA1c, mean (SD), % | 6.0 (2.2) | 6.0 (1.3) | .939 |
| C-reactive protein, mean (SD), mg/dL | 0.66 (1.16) | 0.73 (0.92) | .879 |
| Echocardiography and CMR | |||
| LVEF by echocardiography, mean (SD), % | 51.8 (8.6) | 49.2 (9.3) | .471 |
| LGE by CMR, mean (SD), % | 20.9 (7.9) | 16.9 (11.8) | .375 |
| Edema by CMR, mean (SD), % | 28.9 (10.0) | 19.6 (12.3) | .051 |
| Myocardial salvage, mean (SD), % | 26.8 (13.5) | 17.4 (17.0) | .135 |
| MVO by CMR | 4 (50) | 11 (48) | .922 |
CK-MB, creatine kinase-MB fraction; CMR, cardiac magnetic resonance imaging; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MVO, microvascular obstruction; SD, standard deviation.
Statistical significance, P<.05. Data are expressed as No. (%) or mean (standard deviation).
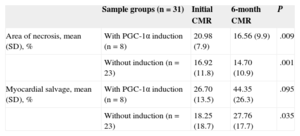
To evaluate the long-time effects of PGC-1α induction during the acute infarction phase, the MS index at 6 months was compared between the 2 groups. Although there was a tendency toward a higher MS index in the PGC-1α induction group (MS percentage at 6 months, 43.1% [SD,9.9%] vs 27.5% [SD,3.5%]; P=.071) (Figure 4), this result was due to the larger area of edema in the acute phase in these patients. Paired sampling analysis of the necrotic area and MS revealed a comparable improvement in both groups over time (Table 3).
Area of Necrosis and Myocardial Salvage: Samples Paired According to PGC-1α Induction in the Acute Phase of Myocardial Infarction. Comparison of Percentages Using a Student t Test for Paired Samples
| Sample groups (n=31) | Initial CMR | 6-month CMR | P | |
|---|---|---|---|---|
| Area of necrosis, mean (SD), % | With PGC-1α induction (n=8) | 20.98 (7.9) | 16.56 (9.9) | .009 |
| Without induction (n=23) | 16.92 (11.8) | 14.70 (10.9) | .001 | |
| Myocardial salvage, mean (SD), % | With PGC-1α induction (n=8) | 26.70 (13.5) | 44.35 (26.3) | .095 |
| Without induction (n=23) | 18.25 (18.7) | 27.76 (17.7) | .035 |
CMR, cardiac magnetic resonance imaging; LGE, late gadolinium enhancement; SD, standard deviation.
Data are expressed as percentages. Statistical significance, P<.05. Area of necrosis estimated by LGE. Myocardial salvage estimated by the difference between the area of the edema in the acute phase and the necrotic area.
However, comparison of the change in the left ventricular volume in these groups at 6 months postinfarction revealed greater VR in the PGC-1α induction group (percentage change in the left ventricular end-diastolic volume, 29.7% [SD,11.2%] vs 1.2% [SD,5.8%]; P=.045) (Figure 3C) and a nonsignificant deterioration of general systolic function (percentage change in the left ventricular ejection fraction, –4.1% [4.2%] vs 5.9% [SD,3.6%]; P=.085) (Figure 3D).
Immune Response and PGC-1αMolecular Activity of PGC-1αSee the supplementary material and the supplementary figure.
Induction of ANT-1 Mediated by PGC-1αMyocardial cellular apoptosis is regulated by ANT-1 in IR conditions with elevated mitochondrial stress, and the expression of ANT-1 is induced in part by PGC-1α. Correlation of the levels of expression of PGC-1α and ANT-1 following STEMI revealed that 7 out of 8 patients with PGC-1α induction following an infarction also showed ANT-1 induction (Table 4A).
Induction of ANT-1 and Ventricular Remodeling According to PGC-1α Induction and the Presence of Microvascular Obstruction
| All patients (n=29)* | With PGC-1α induction (n=8) | Without PGC-1α induction (n=21) | ||
|---|---|---|---|---|
| A | Induction of ANT-1 | More than 2-fold induction of ANT-1 (n=15) | 7 | 8 |
| Less than 2-fold induction of ANT-1 (n=14) | 1 | 13 |
| All patients (n=31) | Induction of PGC-1α (n=8) | Without induction of PGC-1α (n=23) | ||
|---|---|---|---|---|
| B | Ventricular remodeling | Increase in LVEDV > 10% (n=15) | 7 | 8 |
| Increase in LVEDV<10% (n=16) | 1 | 15 |
| All patients (n=31) | MVO (n=15) | Without MVO (n=16) | ||
|---|---|---|---|---|
| C | Ventricular remodeling | Increase in LVEDV > 10% (n=15) | 8 | 7 |
| Increase in LVEDV<10% (n=16) | 7 | 9 |
| All patients (n=31) | Induction of PGC-1α + MVO (n=4) | Without joint association (n=27) | ||
|---|---|---|---|---|
| D | Ventricular remodeling | Increase in LVEDV > 10% (n=15) | 4 | 11 |
| Increase in LVEDV<10% (n=16) | 0 | 16 |
ANT-1, adenine nucleotide translocase 1; LVEDV, left ventricular end-diastolic volume; MVO, microvascular obstruction.
Because patients with PGC-1α induction showed greater left ventricular end-diastolic volume at the 6-month follow-up and because microvascular obstruction (MVO) is an emerging prognostic factor for VR, we decided to assess the predictive ability of PGC-1α analysis after STEMI, taking as a reference the presence of MVO in the initial cardiac magnetic resonance examination. Signs of VR at follow-up were found in 7 out of 8 patients (87.5%) with PGC-1α induction following STEMI (Table 4B) in comparison with 8 of 15 patients (53.3%) with MVO in the initial cardiac resonance imaging (Table 4C).
In combined analysis of MVO and PGC-1α induction following myocardial infarction, the association of the 2 factors perfectly correlated with the presence of VR in the follow-up study (Table 4D).The absence of MVO and PGC-1α induction was more frequent in patients with smaller infarctions, in conjunction with a significant improvement in left ventricular ejection fraction at 6 months and tendency toward preservation of the left ventricular end-diastolic volume (Table 5).
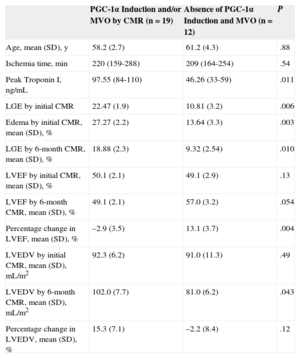
Sample Characteristics According to PGC-1α Induction and the Presence of MVO After STEMI in the Initial CMR Examination: CMR Evaluation of LVEF, LVEDV, and Infarct Size (Estimated by Peak Values of TnI and Area of Necrosis Using LGE)
| PGC-1α Induction and/or MVO by CMR (n=19) | Absence of PGC-1α Induction and MVO (n=12) | P | |
|---|---|---|---|
| Age, mean (SD), y | 58.2 (2.7) | 61.2 (4.3) | .88 |
| Ischemia time, min | 220 (159-288) | 209 (164-254) | .54 |
| Peak Troponin I, ng/mL | 97.55 (84-110) | 46.26 (33-59) | .011 |
| LGE by initial CMR | 22.47 (1.9) | 10.81 (3.2) | .006 |
| Edema by initial CMR, mean (SD), % | 27.27 (2.2) | 13.64 (3.3) | .003 |
| LGE by 6-month CMR, mean (SD), % | 18.88 (2.3) | 9.32 (2.54) | .010 |
| LVEF by initial CMR, mean (SD), % | 50.1 (2.1) | 49.1 (2.9) | .13 |
| LVEF by 6-month CMR, mean (SD), % | 49.1 (2.1) | 57.0 (3.2) | .054 |
| Percentage change in LVEF, mean (SD), % | –2.9 (3.5) | 13.1 (3.7) | .004 |
| LVEDV by initial CMR, mean (SD), mL/m2 | 92.3 (6.2) | 91.0 (11.3) | .49 |
| LVEDV by 6-month CMR, mean (SD), mL/m2 | 102.0 (7.7) | 81.0 (6.2) | .043 |
| Percentage change in LVEDV, mean (SD), % | 15.3 (7.1) | –2.2 (8.4) | .12 |
CMR, cardiac magnetic resonance imaging; LGE, late gadolinium enhancement; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; MVO, microvascular obstruction; SD, standard deviation; STEMI, ST-segment elevation acute myocardial infarction; TnI, troponin I.
Statistical significance, P<.05. Unless otherwise indicated, values indicate mean (interval).
The master transcription factor PGC-1α regulates mitochondrial and nuclear gene expression in cardiac tissue. In addition to its role in energy metabolism, PGC-1α activates mitochondrial biogenesis and coordinates cellular defense against oxidative stress.19
Because endothelial dysfunction caused by reactive oxygen species is an early characteristic of cardiovascular diseases, clinical interest in PGC-1α is due to its central role in intracellular detoxification in conditions such as that of myocardial ischemia.
Although PGC-1α induction has shown beneficial effects in adaptation of neuronal tissue and skeletal muscle to ischemia and intense exercise, respectively, the role of PGC-1α in the stress response in the adult heart remains controversial. In animal models, although cardiotoxic effects follow PGC-1α induction, its genetic deletion causes cardiac dysfunction. Similarly, clinical studies have reported conflicting results regarding the amount of PGC-1α expressed by dysfunctioning cardiomyocytes.20,21 Based on this apparent paradox, other studies have revealed that moderate and transient overexpression of PGC-1α can have beneficial effects in stressful conditions.22 Thus, cell survival in the face of IR injury would depend on the initial degree of activation of the mitochondrial defense system, the magnitude of its response to the oxidative damage, and the duration of the response after the stimulus.
In the present study, PGC-1α expression was assessed in patients with STEMI who underwent reperfusion and, consequently, had IR injury in cardiac tissue. First, the degree of baseline systemic activation was found to be important for the extent of myocardial necrosis, with patients with detectable constitutive expression of PGC-1α in the first blood sample showing a higher MS index at the 6-month follow-up. Moreover, a tendency was seen toward less VR and greater recovery of systolic function. The absence of baseline PGC-1α expression was more frequently found in diabetic patients, although this association was nonsignificant due to the small sample size. These results support the evidence indicating that the integrity of the system regulated by PGC-1α is essential for combating acute IR injury, and could partly explain the worse clinical course of patients with carbohydrate metabolism disorders, given the loss of PGC-1α activity typically seen in patients with diabetes mellitus.23 The AleCardio trial has recently evaluated the effect of the dual PPAR agonist aleglitazar on cardiovascular event reduction following acute coronary syndrome in patients with diabetes. However, the study was prematurely terminated on the recommendation of the safety committee due to a lack of efficacy and an increase in safety end points in an unplanned interim analysis.24
Analysis of the prognostic implication of PGC-1α induction in patients with STEMI showed a correlation between PGC-1α induction and the development of greater VR, although the sample groups were similar in ischemic times, reperfusion strategies, arterial event cause, and initial necrotic area estimated by cardiac magnetic resonance imaging. This increase in the left ventricular end-diastolic volume at the 6-month follow-up was accompanied by a nonsignificant deterioration in systolic function. Induction of PGC-1α was also followed by significantly increased expression of its gene targets cytochrome C and GPx. These data support the hypothesis that excessive induction, both of PGC-1α and the PGC-1α–regulated mitochondrial system that protects against oxidative stress, would have negative effects on cell survival in IR conditions. The causal physiopathological mechanism could partly be explained by IR injury triggering PGC-1α–mediated induction of ANT-1, which increases cell apoptosis in a dose-dependent manner in various tissues, including heart tissue.25
It has recently been demonstrated that cellular resistance to stress is decreased by coordinated activation of ANT-1 and PGC-1α in mouse models of ischemia.16 Due to the evidence of contractile dysfunction following PGC-1α induction in IR conditions,14 ANT-1 has been proposed to also participate in the development of this adverse phenotype, although this situation has not been clinically demonstrated in patients. In the present study, comparison of the groups according to PGC-1α induction following STEMI revealed a greater induction of ANT-1 in patients with PGC-1α induction. Thus, these results would be the first to support the hypothetical role for ANT-1 in a clinical scenario of IR injury.
Due to the correlation between PGC-1α induction and VR development, the prognostic ability of PGC-1α induction was also compared, taking as a reference the presence of MVO on cardiac magnetic resonance imaging, already demonstrated to be an excellent prognostic factor for VR.26,27 In our study, the predictive capacity of PGC-1α induction following STEMI was superior to that of the presence of MVO, and the joint finding of both variables perfectly correlated with the onset of VR in all patients. In contrast, the absence of MVO and PGC-1α induction was associated with better clinical outcome, both for preservation of left ventricular end-diastolic volume and for improvement in left ventricular ejection fraction. Even with the limitations caused by the small number of patients, these data indicate the prognostic value of PGC-1α induction for myocardial recovery after STEMI.
In summary, the evidence derived to date from basic research shows that PGC-1α regulates the cellular protection system against IR injury, with uncertain results for its role in cardiac tissue. Our work monitored the systemic expression of PGC-1α after STEMI in a clinical setting in order to define a cardioprotective activation profile. Thus, whereas constitutive PGC-1α expression was associated with greater MS, its immediate induction after STEMI correlated with greater VR in the postinfarction course. This damaging effect on cardiac recovery could be partly explained by PGC-1α–mediated induction of ANT-1.
All together, these data indicate that the integrity of the mitochondrial protection system regulated by PGC-1α is essential for an adequate response to IR injury, whereas its excessive and persistent activation after an ischemic event has damaging effects on cardiac recovery. However, the prognostic implications of these findings are beyond the scope of this work.
Study LimitationsThe main limitations of the present study are the small sample size and the eminently basic character of the research. Thus, although the translational design of this study allows a working hypothesis to be established based on the conclusions, future clinical studies are required to confirm the findings and define the role of PGC-1α measurement in clinical practice.
CONCLUSIONSSystemic expression of PGC-1α can be monitored in patients with STEMI. Whereas a constitutive increase in the activity of the system regulated by PGC-1α correlated with greater MS, excessive and persistent induction of PGC-1α after STEMI was associated with greater VR. This damaging effect on cardiac recovery could be related to PGC-1α–mediated induction of ANT-1, which promotes cellular apoptosis in the IR lesion.
FUNDINGThis work was financed by the Spanish Society of Cardiology through a grant for Proyectos de Investigación Básica en Cardiología, 2010; the Programa I3 de Intensificación Investigadora 2010 and 2012 from the Instituto de Salud Carlos III and the Generalitat Valenciana; the Fundación para la Investigación del Hospital General Universitario de Valencia through the Becas de Investigación Intramural, 2008 and 2010; the Spanish Ministry of Science and Innovation through projects SAF 2009-07599 and CSD 2007-00020; SAF2012-37693 from MINECO (Ministerio de Economía y Competitividad); and S2010/BMD-2361 from the Community of Madrid.
CONFLICTS OF INTERESTSNone declared.