Left ventricular false tendon is a structure of unknown function in cardiac physiology that was first described anatomically by Turner. This condition may be related to various electrical or functional abnormalities, but no consensus has ever been reached. The purpose of this study was to determine the time of appearance, prevalence and histologic composition of false tendon, as well as its association with innocent murmur in children and with heart disease.
MethodsThe basic research was performed by anatomic dissection of hearts from adult human cadavers to describe false tendon and its histology. The clinical research consisted of echocardiographic study in a pediatric population to identify any relationship with heart disease, innocent murmur in children, or other abnormalities. Fetal echocardiography was performed prenatally at different gestational ages.
ResultsFalse tendon was a normal finding in cardiac dissection and was composed of muscle and connective tissue fibers. In the pediatric population, false tendon was present in 83% on echocardiography and showed a statistically significant association only with innocent murmur in children and slower aortic acceleration. The presence of false tendon was first observed on fetal echocardiography from week 20 of pregnancy.
ConclusionsLeft ventricular false tendon is a normal finding visualized by fetal echocardiography from week 20 and is present until adulthood with no pathologic effects except for innocent murmur during childhood. It remains to be determined if false tendon is the cause of the murmurs or if its absence or structural anomalies are related to disease.
Keywords
Left ventricular false tendon (FT) was first described in 1893 by Dr Turner, who observed it on dissection of the human heart.1 This fibromuscular structure originates in the interventricular septum and crosses the left ventricle to the papillary muscles, lateral wall, or cardiac apex. Since that time, the structure has been studied by human2–4 and animal5,6 cadaver dissection. Technological breakthroughs have also led to other techniques, such as echocardiography7–11 (including 3-dimensional)12 and magnetic resonance imaging,13,14 that permit visualization of live human hearts. The percentage of FT visualization in humans has been rising with technical improvements: the earliest studies report rates of 0.5% whereas current investigations have achieved up to 78%.7–12,15–17 False tendon has been related to clinical signs, such as innocent murmur,16–18 conduction and heart rate abnormalities,19,20 cavitary thrombi,21 and even infections,22 although there is no consensus on the implication of FT in human physiology or disease. Its morphogenesis and embryonic origin are not well known, and prenatal imaging has been rare, with only a few reports of the condition observed during the fetal period.23,24 Our study sought further information on the gestational age at which FT can first be seen, the implications of its presence during childhood, the actual prevalence in adults by postmortem studies, and the histologic characteristics of the structure.
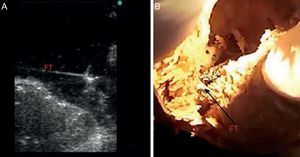
METHODSBasic ResearchAnatomic StudyThe basic anatomic study was designed to determine the actual prevalence of FT in human hearts by anatomic dissection. We selected 41 human cadavers (age range, 65-88 years; 18 women/23 men) from voluntary donors between January and November 2012. The bodies were previously fixed by perfusion through a cannula implanted in the common carotid artery and then preserved in ponds of 5% formaldehyde solution. The hearts were extracted by thoracotomy, labeled from #1 to #41, weighed on a precision scale, and measured (length and width) with a caliper. The interventricular septal and lateral wall thicknesses were also measured. A scalpel was used to make incisions on the lateral wall of the left ventricle to visualize the interior of the left ventricle and FTs, with FT anatomically defined as fibromuscular structures originating at the interventricular septum that cross the left ventricle to the papillary muscles, lateral wall, or cardiac apex. The number, location, and thickness of the FTs were studied; 10 of the dissected hearts with a visible FT were submerged in a basin with water for direct ultrasound of the structure (Figure 1).
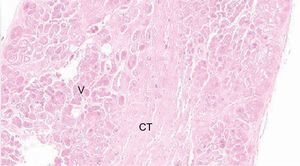
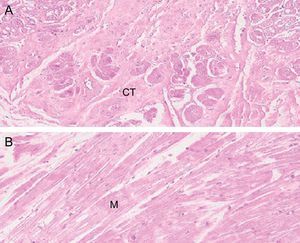
HistologyFalse tendon specimens were obtained for histological examination from 5 of the 41 hearts studied. In this case, the specimens were preserved in formaldehyde, then sliced and stained with hematoxylin-eosin and Masson trichrome. We obtained microphotographs at ×10, ×20, and ×40 (Figure 2).
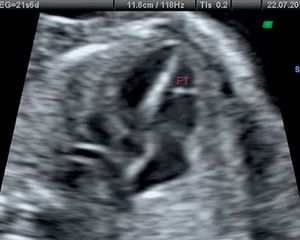
Clinical ResearchClinical Study in the Pediatric PopulationThe clinical population study was designed primarily to identify any statistical association between the presence of FT visualized on echocardiographic study and the presence of innocent murmur in children, defined as grade II/VI or lower heart murmur and vibratory or musical characteristics auscultated in children, predominantly in the left sternal borders and with no other accompanying signs or symptoms. On echocardiography, FT was defined as the structure located in the left ventricle and visualized as a linear echo-refringent component originating in the lateral ventricular wall, cardiac apex, or papillary muscles and reaching the interventricular septum. The sample size was estimated at 150 patients to estimate prevalence with a 95% confidence interval (95%CI), precision of 7.3%, and loss rate of 10%, assuming 75% with the presence of FT, a value taken from similar previous studies. The inclusion criteria were patients aged 0 to 14 years seen for the first time by a pediatric cardiologist and referred by the primary or in-hospital outpatient clinic. The exclusion criteria were lack of parental consent, previous ultrasound performed by the observer, or assistance with body measurements and electrocardiogram before the ultrasound. The echocardiography was performed by a pediatric cardiologist experienced in this technique who adhered to the pediatric clinical guidelines and echocardiography standards,25 using a Philips HD 4000 ultrasound unit with 8- and 4-MHz transducers. The study was approved by the Research Ethics Committee of the Hospital Universitario de San Juan. The study protocol included a complete medical history from the patient, body measurements, electrocardiogram, and echocardiography.
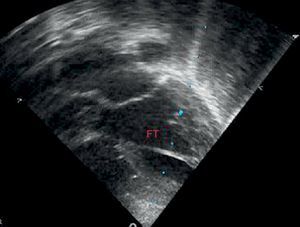
Statistical MethodsThe data were collected and analyzed by SPSS, which was used to study all analyzed data from the medical history and additional examinations (body measurements, electrocardiogram, and echocardiography). Data were collected on the presence or absence of FT, number of FTs, thickness, and location in the left ventricle (divided into 3 similar parts in the apical, medial, and superior thirds) (Figure 3). SPSS was also used for the descriptive, bivariate, and multivariate analyses. The descriptive analysis was performed using absolute and relative frequencies for qualitative variables and mean (standard deviation) for quantitative variables. The bivariate analysis was performed using chi-squared and Student t tests, as applicable in each case. In addition, the raw odds ratios (OR) were calculated. Lastly, a multivariate logistic regression model was fitted to control for possible confounding variables. All analyses were performed with a significance value of 5%, and the confidence interval was obtained for each parameter.
Clinical Study in Pregnant WomenLastly, fetal echocardiography was proposed to pregnant women in gestational weeks 12 to 32, with the aim of visualizing any FTs. The study was performed in 10 pregnant women by gynecologists with specific fetal echocardiography training in a fetal diagnostic unit in a tertiary hospital.
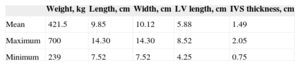
RESULTSBasic ResearchAnatomic StudyInitially, the basic measurements were taken from the hearts listed in Table 1. The results of cadaver dissection of human hearts showed that 41 hearts had at least 2 (mean, 3.7) FTs. We divided them according to their visual characteristics of thickness in the largest FTs (muscular, M) and finest (tendinous, T), the latter being shorter and finer (Table 2).
Summary of the General Characteristics of the Hearts Studied by Cadaver Dissection
| Weight, kg | Length, cm | Width, cm | LV length, cm | IVS thickness, cm | |
|---|---|---|---|---|---|
| Mean | 421.5 | 9.85 | 10.12 | 5.88 | 1.49 |
| Maximum | 700 | 14.30 | 14.30 | 8.52 | 2.05 |
| Minimum | 239 | 7.52 | 7.52 | 4.25 | 0.75 |
IVS, interventricular septum; LV, left ventricle.
Results for False Tendon From Cadaver Dissection
| Number of FTs | FT length, mm | FT thickness, mm | |
|---|---|---|---|
| Mean | 3.7 | 1.84 (T)/2.21 (M) | 0.056 (T)/0.28 (M) |
| Maximum | 7 | 4.04 (T)/5.54 (M) | 0.22 (T)/0.85 (M) |
| Minimum | 2 | 0.54 (T)/0.61 (M) | 0.01 (T)/0.05 (M) |
FT, left ventricular false tendon; M, FT of muscular characteristics; T, FT of tendinous characteristics.
The most common location was the region closest to the cardiac apex. Less than 5% of FTs were found in the third farthest from the apex (Figure 4).
Histology ResultsThe histology of the fixed and studied FTs showed muscle fibers and connective tissue of various proportions, depending on the location of the slice. Vascularization of the FTs was evident in the slices (Figure 5).
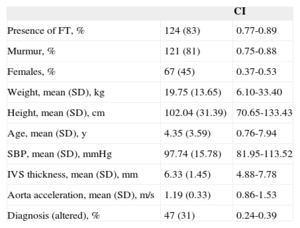
Clinical Study OutcomesClinical Study Outcomes in the Pediatric PopulationHeart murmur was observed in 81% (95%CI, 0.75-0.88) of children attending the cardiology outpatient clinic and FT was seen in 83% of these patients (Table 3). Among the patients with FT, 75% had 1 FT, 24% had 2, and only 1% had 3 or more. The site was usually the middle region (67%), followed by the cardiac apex (22%) or upper third (11%). No differences were observed in the presence or absence of FT according to age, sex, weight, height, blood pressure, interventricular septal thickness, or the presence of heart disease, which we defined as electrocardiographic or morphologic abnormality in the echocardiographic study. Conversely, the FTs showed no significant relationship with innocent murmur in children and slower descending aorta acceleration (P<.05) (Table 4).
Description of the Pediatric Population Study
| CI | ||
|---|---|---|
| Presence of FT, % | 124 (83) | 0.77-0.89 |
| Murmur, % | 121 (81) | 0.75-0.88 |
| Females, % | 67 (45) | 0.37-0.53 |
| Weight, mean (SD), kg | 19.75 (13.65) | 6.10-33.40 |
| Height, mean (SD), cm | 102.04 (31.39) | 70.65-133.43 |
| Age, mean (SD), y | 4.35 (3.59) | 0.76-7.94 |
| SBP, mean (SD), mmHg | 97.74 (15.78) | 81.95-113.52 |
| IVS thickness, mean (SD), mm | 6.33 (1.45) | 4.88-7.78 |
| Aorta acceleration, mean (SD), m/s | 1.19 (0.33) | 0.86-1.53 |
| Diagnosis (altered), % | 47 (31) | 0.24-0.39 |
CI, confidence interval; FT, left ventricular false tendon; IVS, interventricular septum; SBP, systolic blood pressure; SD, standard deviation.
Unless otherwise indicated, the data are expressed as No. (%).
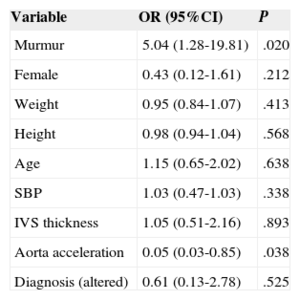
Multivariate Analysis Showing That the Presence of False Tendon Shows a Statistically Significant Association With Murmurs and Aortic Acceleration
| Variable | OR (95%CI) | P |
|---|---|---|
| Murmur | 5.04 (1.28-19.81) | .020 |
| Female | 0.43 (0.12-1.61) | .212 |
| Weight | 0.95 (0.84-1.07) | .413 |
| Height | 0.98 (0.94-1.04) | .568 |
| Age | 1.15 (0.65-2.02) | .638 |
| SBP | 1.03 (0.47-1.03) | .338 |
| IVS thickness | 1.05 (0.51-2.16) | .893 |
| Aorta acceleration | 0.05 (0.03-0.85) | .038 |
| Diagnosis (altered) | 0.61 (0.13-2.78) | .525 |
95%CI, 95% confidence interval; IVS, interventricular septum; OR, odds ratio; SBP, systolic blood pressure; SD, standard deviation.
In the multivariate analysis, the adjusted OR (AOR) was 5.05 (95%CI, 1.28-19.81) for the presence of murmur and 0.05 (95%CI, 0.03-0.85) for descending aorta acceleration, such that the presence of FT was related to slower velocity in the descending aorta (Table 4).
Study Outcomes in Pregnant WomenTen echocardiograms were performed and showed FT in 6 fetuses from week 20 to week 36. False tendon was not visualized before week 20 (Figure 6).
DISCUSSIONAlthough FT was described some time ago and can readily be observed on routine additional tests (echocardiography5,7–12 and magnetic resonance imaging13), it has not been clearly established if the structure is normal or if it is related to any diseases. Population studies have been performed by autopsy or 2-dimensional ultrasound.2–12 The implication of FT for cardiac physiology is still unknown, and its association with some heart diseases19–21,26,27 has not been well determined; hence, there is no consensus that the finding has clinical implications. For this reason, we decided to undertake a complete review of FT by focusing our study on 4 fields to determine its prevalence and histologic, anatomic, and echocardiographic characteristics during the fetal stage and childhood years in order to understand if the entity correlates with heart disease.
The results obtained from cadaver dissection of human hearts show that the presence of FT is the norm without exception and that a mean of 3 to 4 FTs are found. This level is the highest of those observed in previous studies,4 as up to 95% had been found in small animal series.16 As in earlier studies, histology showed that FTs are composed of collagen and muscle fibers in different percentages, although muscle fibers are more frequent in thicker FTs. This allowed them to be classified into 2 groups (muscular and tendinous), although it is not known if they are functionally different. Staining did not allow the electric transmission structures to be visualized, although some studies do report that they are present and implicated in cardiac arrhythmias.19,20,26
The pediatric population study found FT on ultrasound in 83% of patients, which is the highest frequency of FT found by ultrasound in published studies to date. This finding can be explained by technical improvements in ultrasound units, as well as the study approach used to visualize the structure. Unlike the dissection studies, not all FTs were found for 2 reasons: 1) FTs are sometimes thin and can go unnoticed and 2) visualization of FTs in the apex may be impossible because the region is hard to examine by echocardiography and most FTs are found during dissection.
Like some previous studies,16–18 a statistically significant association was shown between the presence of FT visualized on echocardiography and innocent murmur in children (P<.05). A lower velocity was also found in the descending aorta. There were no other relationships with echocardiography findings, electrocardiography abnormalities, or clinical symptoms that could be statistically related to the presence of FT. There have been reports of multiple associations between the presence of FTs and the origin of arrhythmias,19,20,26 valve abnormalities,21 and infections,22 although these associations have not been clearly demonstrated. As in this study, the most widely accepted association is with innocent murmur in children.16–18 This murmur has been related to increased velocity in the aorta28; in our study, the presence of FT was not a confounding factor but rather indicated that this velocity is slower. In fact, the movement produced by blood outflow may produce vibratory movement in the FT that causes the murmur and reduces blood flow at the aortic velocity. The factors of FT length, thickness, ejection volume, and left ventricular size would mean that the ideal factors for its presence occur in childhood.
Logically, if FT is present from birth, it must originate during cardiac organogenesis, as shown in basic studies on noncompacted cardiomyopathy that have reported the formation of these structures between weeks 5 and 8 of pregnancy.29,30 Fetal echocardiography has demonstrated the presence of FTs as of week 20,23,24 and our study also found FT to be present from week 20. Although FT is presumed to exist at earlier ages according to certain studies,29,30 current methodological ultrasound techniques are unable to visualize the condition. The time at which FT appears cannot be established, but it must originate from the time of organogenesis.
CONCLUSIONSOur study investigated FT from various perspectives and found that the structure is present from the fetal stage into adulthood and that its presence in pediatric echocardiography is not related to heart disease. This structure did exhibit a statistical association with innocent murmur in children, but causality was not shown because the mechanism is still not described and demonstrated. Although FT is a normal finding, it cannot be ruled out that anomalous or missing FT could be related to disease, and further studies are required on this topic.
CONFLICTS OF INTERESTNone declared.