Keywords
INTRODUCTION
Acute cellular rejection causes morbidity and cell loss in the graft following heart transplantation, and requires invasive monitoring involving the performance of serial endomyocardial biopsies.1
Apoptosis, or "programmed cell death" is implicated in several different heart diseases, and the results observed in terms of the existence of a possible relationship between apoptosis and acute cellular rejection are disparate.2-10 In experimental studies in rats, myocyte apoptosis was detected in allografts, but not in homografts,2,3 although Bergese et al4 concluded that apoptosis was not indicative of acute rejection; rather, it was related to chronic rejection. With respect to human heart transplantation, Laguens et al5 studied 63 endomyocardial biopsies from six recipients and found myocyte apoptosis more frequently in specimens exhibiting higher-grade rejection. Jollow et al6 studied 22 biopsy specimens showing moderate rejection, and found no myocyte apoptosis, detecting only that of mononuclear cells. Szábolcs et al7 analyzed 30 biopsy specimens with 2R/3R rejection, comparing them with 12 without rejection, and found a 30-fold increase in apoptotic cardiomyocytes in the first group. Puig et al,8 by means of a histological study and isotopic labeling of 40 biopsy specimens, observed cardiomyocyte apoptosis more frequently in severe rejection, but also in specimens in which there was no histological evidence of rejection. Masri et al,9 in their evaluation of 59 biopsy specimens from 24 patients, detected increased caspase activity and apoptosis predominantly of infiltrating cells during acute rejection, which persisted after its histological resolution. Koch et al10 examined biopsy specimens from 27 recipients and detected apoptosis nearly exclusively of interstitial cells, which were present in higher numbers and showed a more marked expression of the antiapoptotic molecule, Bcl-2, at higher levels of rejection; they detected nearly no apoptosis in cardiomyocytes, and there were no differences in the expression of Bcl-2. With respect to the mechanisms involved in apoptosis in heart transplantation, studies have been carried out to determine the role of nitric oxide through nitric oxide synthase (NOS),7,11 of the Fas/FasL system3,12,13 and of the Bax/Bcl-2 family of mediators,10,14 among others.
The verification of the possible involvement of apoptosis in the pathophysiology of acute cellular rejection and the investigation of the molecular pathways involved could open a door to new diagnostic and therapeutic approaches to rejection. We applied various techniques for the detection of apoptosis to the myocardial tissue and serum of heart transplant recipients for the purpose of: a) assessing the existence of cardiomyocyte apoptosis in the transplanted human heart as a mechanism of cell loss and its progression over time; b) studying the possible association between cardiomyocyte apoptosis and acute cellular rejection; and c) analyzing the possibility of the noninvasive detection of rejection by establishing serological markers of apoptosis.
Cellular apoptosis has regulatory mechanisms that involve a large number of intracellular mediators. It can be triggered in 2 ways: extrinsically and intrinsically. Both in combination, take part in the activation of the caspases, a family of intracellular proteases that act stepwise and ultimately produce the morphological changes in the cell.15-17 We selected, for evaluation in human heart transplantation, variables that act at each of the three levels of the temporal sequence of the process of programmed cell death: soluble Fas, tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6) as serum parameters related to the triggering of apoptosis via the extrinsic pathway; in addition, we employed the detection of caspase 3 as an enzyme that could play a major role in the process and TdT-mediated dUTP nick end labeling (TUNEL) as an established detector of myocyte apoptosis.
METHODS
Study Design
In this prospective clinical cohort study, we included consecutively all the patients who underwent heart transplantation in our unit over a 6-month period (n=14 patients). The exclusion criteria were death during the initial postoperative period and retransplantation.
Endomyocardial biopsies were performed within the first six months after the transplantation, the period during which the incidence of acute rejection is highest (n=130 biopsies; 7-12 per patient). In the first 10 patients, serum samples were obtained at the time of the biopsy (n=80 samples); this was not carried out in the remaining 4 patients due to limitations in the availability of reagents.
A prospective clinical follow-up was carried out for 4 years from the moment of inclusion, according to the usual clinical protocol employed in our unit, and data was collected on the recipient, the donor, the procedure, the immunosuppressive regimen and the clinical course.
Study Variables
In the biopsy specimens, we determined the grade of acute cellular rejection (International Society for Heart and Lung Transplantation [ISHLT]), assessed cardiomyocyte apoptosis using the TUNEL technique and carried out the detection of caspase
3. In the serum samples, we quantified soluble Fas, TNFα, and IL-6.
Sample Collection and Processing
We followed the protocol for monitoring rejection currently employed at the time of the study (first biopsy 10 to 12 days after transplantation, every 10 days during the first 2 months, every 15 days during months 3 and 4, and once a month during months 5 and 6).
The biopsies were obtained according to the usual protocol, and 4 to 6 endomyocardial specimens measuring 2 to 3 mm in diameter were obtained from the right ventricle of each patient per procedure. They were fixed in a 10% buffered formaldehyde solution (Sigma, St. Louis, Missouri, United States) and were maintained for 30 minutes in heated formol (70ºC). After being embedded in paraffin, the tissue was cut (3-4 µm) with a Jung RM 2035 microtome (Leika, Spain), and was placed on a microscope slide. The specimens were considered to be sufficient in number when there were at least 3 evaluable fragments, once areas of fibrosis detected in a previous biopsy had been excluded.
Eighty blood samples were drawn from the first 10 patients included, on the same days as the collection of the biopsy specimens. The samples were processed to obtain serum.
Determination of the Grade of Rejection
The samples were stained with hematoxylin-eosin and Masson's trichrome. The grade of rejection was determined according to the ISHLT classification in effect when the study was designed: 0, absence of rejection; 1A, focal infiltrate without necrosis; 1B, diffuse infiltrate without necrosis; 2, a single focus of aggressive infiltration and/or focal myocyte damage; 3A, multifocal aggressive infiltrates and/or myocyte damage; 3B, diffuse inflammatory infiltrates with necrosis; 4, diffuse polymorphous inflammatory infiltrate, with edema, hemorrhage, vasculitis and necrosis. The current ISHLT classification is as follows: 0R=0; 1R=A, 1B and 2; 2R=3A; 3R=3B and 4.
Detection of Myocyte Apoptosis by Means of TUNEL
The TUNEL technique is based on the labeling and detection of the 3'-OH terminal characteristically exposed upon the fragmentation of DNA during the apoptotic process. It detects the nuclei of apoptotic cells in histological sections without destroying the tissue integrity.
The binding of said DNA fragments to fluorescein-labeled deoxyuridine triphosphate (dUTP) was carried out in a reaction mediated by deoxynucleotidyl transferase (TdT) (Boehringer Mannheim, In Situ Cell Death Detection Kit, POD). The tissue sections were deparaffinized with xylene and rehydrated with alcohol, introduced into a 0.1 mol citrate solution and microwaved for 5 minutes. They were then washed and covered with the TUNEL mixture, which contained TdT and fluorescein-dUTP, and incubated in a humidity chamber for 1 hour at 37ºC, and subsequently with an antibody specific to fluorescein conjugated with peroxidase. The utilization of diaminobenzidine made it possible to view the brown-colored labeled nuclei. The nuclei without DNA fragmentation were stained with hematoxylin. In every case, a sample of human palatine tonsils obtained at the time of the biopsies was processed as control (we know that apoptosis is commonly produced in the lymphoid follicles of the tonsil).
Each tissue section was examined under a light microscope at magnifications of 25, 100, and 400. In every sample, 10 fields were randomly selected for examination, the number of cardiomyocytes found to be positive in the TUNEL analysis was quantified and the mean value was defined as the apoptotic index.
Histochemical Detection of Caspase 3 Activity
It is thought that caspase 3 (CPP32) could play a major role in apoptosis. We analyzed the degree of expression of CPP32 in the endomyocardium by means of histochemical techniques. For this purpose, after deparaffinization and rehydration, the tissue sections were incubated with horse serum for 30 minutes at room temperature, washed with buffered saline and stained with a specific mouse monoclonal antibody, which was employed at a dilution of 1:1000. As negative controls, the technique was performed in several samples without the antibody. Each tissue section was examined by light microscopy at magnifications of 25, 100, and 400, and was assessed in terms of the positivity or negativity in cardiomyocytes according to the technique described.
Determination of Soluble Fas in Serum
Fas and Fas ligand (FasL) are cell surface proteins and both have a soluble form. Fas induces apoptosis when it binds to FasL or to the soluble form of FasL. Soluble Fas, which lacks the transmembrane portion of Fas, blocks apoptosis because it inhibits the binding of Fas to FasL or to soluble FasL. Its determination in serum is carried out by means of enzyme-linked immunoabsorbant assay (ELISA) (sAPO-1/Fas ELISA, Zymed Laboratories, Inc).
Determination of Tumor Necrosis Factor Alpha in Serum
Tumor necrosis factor alpha induces apoptosis through the binding of its specific cell membrane receptor, TNF-R1, triggering the extrinsic pathway. Its determination is carried out in serum by means of ELISA (Quantikine HS Human TNFα).
Determination of Interleukin 6 in Serum
Interleukin 6 is an inducer of apoptosis related to the Fas-FasL system, which induces the transcription of the Fas/APO 1 receptor gene. Its determination is performed in serum using ELISA (Quantikine HS Human IL-6).
The concentration of each soluble product was calculated by extrapolating the optical density of each study sample from a reference curve created with the optical densities of different samples of known concentrations of each product. All the determinations were performed in duplicate.
Statistical Analysis
The results of the variables were recorded, with the mean, median, standard deviation, minimum and maximum in each patient. The comparison of the means obtained in the patients for the parameters analyzed was carried out using nonparametric analysis of variance (Kruskal-Wallis test) for the purpose of identifying differences in variability among patients; if this variability was significant, the variable was not analyzed in subsequent analyses. The hypothesis of a normal distribution of the data was assessed using the Shapiro-Wilk test. Given that the variables corresponding to the samples did not follow a normal distribution, the nonparametric Spearman correlation coefficient and its hypothesis test were used to evaluate the degree of correlation. The assessment of diagnostic capability to detect rejection of the serological variables studied was carried out by creating receiver operating characteristic (ROC) curves, and summarized in the area under the curve (95% confidence interval [CI]) and the hypothesis test. The changes in the different parameters over the course of time were examined. In all cases, the differences were considered to be statistically significant when the P value was <.05. The statistical study was carried out using the SPSS software package (v. 10.0) for Windows operating system.
RESULTS
Fourteen patients were included (12 men; mean age, 50 [8.4] years). The heart diseases that led to the need for transplantation were: ischemic cardiomyopathy (n=7 patients), idiopathic dilated cardiomyopathy (n=6), aortic insufficiency (n=1), and alcohol-related heart disease (n=1). The donors were men between 21 and 52 years of age. All of the patients received induction immunosuppressive therapy with OKT3 and maintenance with cyclosporine, azathioprine and steroids, except in one case, in which mycophenolate was used in place of azathioprine. One patient died suddenly in the seventh month after transplantation due to an infectious process; the remainder exhibited a good functional status at the end of follow-up. Eleven patients had one or more episodes of 2R rejection or worse and received specific treatment.
The distribution of the 130 biopsy specimens according to the grade of rejection was as follows: 0R, n=62; 1R, n=39 (1A, n=12; 1B, n=12; 2, n=15); 2R, n=27; and 3R, n=2 biopsies.
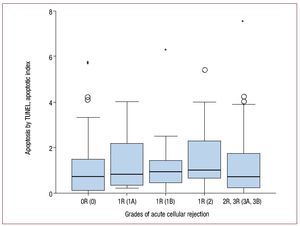
Apoptotic cardiomyocytes were detected by means of TUNEL in 106 of the 130 biopsy specimens, a fact that confirms the existence of apoptosis in the transplanted heart. The mean apoptotic index was 1.2 (1.48). No significant correlation was observed between the TUNEL values and the grade of rejection (rs=0.08; P=.37) (Figure 1). TUNEL-positive myocytes were detected in biopsy specimens in which rejection was not present.
Figure 1. Levels of apoptosis detected by TUNEL for each grade of rejection (n=130 biopsies). Grades of rejection according to the ISHLT (previous classification in parentheses). Apoptosis is graded according to the "apoptotic index," obtained as explained in the text. In the graph, the box limits are the 25th and 75th percentiles; the median is shown in black, and the maximum and minimum values appear outside the limits, provided they surpass by 1.5-fold the distance between the corresponding quartile and the closure limit (the values represented by asterisks are the furthest outlying values, surpassing the indicated distance by 3-fold).
Of the samples examined, 30 were positive for the histochemical detection of caspase 3 and 68 were negative (Table). The remaining samples were considered unevaluable or the available sample was insufficient. No correlation was found between caspase 3 positivity and the rejection grade or the results of TUNEL analysis.
No significant correlation was observed between the grade of rejection and the soluble Fas levels (rs=0.44; P=.7) (Figure 2).
Figure 2. Soluble Fas levels (U/mL) for each grade of rejection (n=80 serum samples). Graphic representation similar to that of Figure 1.
The comparison of IL-6 levels using analysis of variance (Kruskal-Wallis) revealed significant differences between patients (P<.005); thus, it was not considered appropriate to continue this assessment with respect to other study variables.
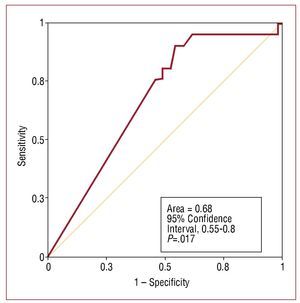
There was a significant negative correlation between the grade of rejection and TNFα levels (rs=-0.333; P=.003) (Figure 3). On the basis of this finding, we evaluated its suitability as a diagnostic test by obtaining the ROC curve. The value of the area under the curve proved to be quite low (0.68) and it was not considered appropriate to establish cut-off points (Figure 4). Our understanding is that the accuracy of the test is too low for it to be useful in the noninvasive diagnosis of rejection.
Figure 3. Serum tumor necrosis factor alpha (TNFα) levels (pg/mL) for each grade of rejection (n=80 serum samples). Graphic representation similar to that of Figure 1.
Figure 4. Assessment of the suitability of tumor necrosis factor alpha (TNFα) for the noninvasive diagnosis of acute rejection. Receiver operating characteristic (ROC) curve.
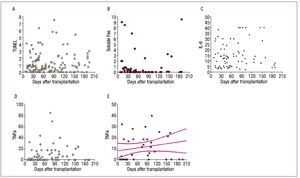
The changes in the variables studied in the TUNEL analysis, soluble Fas and IL-6, did not follow a temporal pattern (Figures 5A-C). When we disregarded those values that coincided with episodes of rejection, TNFα tended to increase slightly from the time of the transplantation (Figure 5E).
Figure 5.Changes in the variables. A-C: TUNEL, soluble Fas and interleukin 6 (IL-6) do not follow any pattern over time. D and E: tumor necrosis factor alpha (TNFα) follows a slightly upward pattern from the time of transplantation if we disregard the values that coincide with rejection episodes.
The variables were assessed in episodes of prolonged rejection, comparing them with periods during which there was a prolonged absence of rejection, defined respectively as grade ≥3A (≥2R) and as grade 0R or that observed in at least 2 consecutive biopsies. The TNFα levels were significantly lower in the cases of prolonged rejection (P=.008). The levels of cardiomyocyte apoptosis tended to be higher during prolonged episodes of rejection, but the difference did not reach statistical significance (P=.168). In the remaining variables, no significant differences were found.
DISCUSSION
The present report is the first longitudinal study on apoptosis in acute cellular rejection following human heart transplantation, with serial data from patients within the same periods of time after transplantation, and follow-up over the course of development. In addition, we selected the variables for the purpose of studying apoptosis at different points throughout its temporal sequence. With respect to the number of biopsies analyzed, this is the most extensive study published in this field. As a result, we have detected cardiomyocyte death due to apoptosis in the transplanted heart, although we were unable to establish a relationship between apoptosis and rejection. Having applied the most widely utilized histochemical method, the TUNEL technique for the detection of the DNA fragments that are typically produced during apoptosis, we found no evidence of a significant correlation with rejection (P=.37). We observed a trend toward higher values in the TUNEL analysis only in prolonged episodes of rejection, but they did not reach statistical significance (P=.16).
A large number of the studies on apoptosis in heart transplantation have been carried out in experimental models in animals,2-4 with results that can be difficult to extrapolate to the complex process of human heart transplantation. The results of those performed in humans5-10 suggest that there could be some correlation between apoptosis and rejection, although it is not corroborated in all the studies, which have involved smaller numbers of samples than those examined by us. The fact that apoptosis was detected in samples in which rejection was not present indicates that there are other causal mechanisms in the cardiac graft.
It is difficult to define the hypothetical interrelationship between apoptosis and rejection in a process as complicated as heart transplantation since, in addition to rejection, multiple stimuli that can cause apoptosis come together: treatment of the donor with catecholamines, ischemia in the organ during the period between harvesting and implantation, extracorporeal circulation and the administration of drugs such as calcineurin inhibitors; in this respect, cyclosporine A inhibits FasL and TNFα, increasing IL-6 and modulating antiapoptotic substances such as Bcl-2 protein.14
Another interesting point is the doubt as to the extent to which we are actually capable of detecting apoptosis with the available techniques. In most of the studies, it has been assessed using TUNEL, which detects the DNA fragments that are typically produced during this process and enables the quantification of the cells by means of light microscopy. It has been proposed that active RNA synthesis and DNA damage in necrotic cells can, in some cases, cause "nonspecific" staining and, in some studies, it has not been possible to verify morphological evidence of apoptosis in cells positive in the TUNEL analysis using electron microscopy.18 One noteworthy observation is the disparity among the percentages of apoptotic cells detected by TUNEL in different studies in heart failure.19,20
Another difficulty for the detection and quantification of programmed cell death could be its short duration, since there remain no traces once the phagocytosis of the apoptotic bodies has been completed; given that the tissue structure remains intact, its total magnitude could be underestimated. Moreover, both rejection and apoptosis are processes that are irregularly distributed in the myocardium and, thus, are subject to a wide sampling variability in the collection of the biopsy specimens.
In our study, we found no correlation between caspase 3 positivity and the grade of rejection. It is not clear whether this enzyme plays a major role in apoptosis in all cell types; some authors affirm that the caspases vary depending on the cell type and even on the stimulus that induces the process.16 It has been little studied in heart transplantation; in one study, the inhibition of caspase 3 appeared to reduce the development of graft vascular disease21; in acute rejection, Masri et al9 found an increased caspase 3 and 8 activity in moderate rejection, which persisted after the resolution.
With respect to the role of the Fas/FasL system, Toyozaki et al22 found higher soluble Fas concentrations in myocarditis than in heart failure or in healthy volunteers. However, a number of studies have reported an increase in soluble Fas in heart failure.23 In heart transplantation, a recent study found an increased expression of Fas and FasL mRNA in biopsies exhibiting acute rejection, but the authors observed no differences in soluble Fas expression with or without rejection.13 We have found no significant correlation between soluble Fas levels and the grade of rejection.
It is a well-established fact that high TNFα concentrations are elevated in patients with heart failure and that this circumstance can have a negative effect on myocardial function. In heart transplantation, from the early stages, an increased expression is observed, higher than the levels encountered in heart failure, which is not associated with contractile dysfunction.24 Chollet et al25 reported that TNFα increases coinciding with rejection in heart transplantation, but the small number of samples employed in the study limits its validity. In contrast, our results appear to indicate that serum TNFα could decrease in higher grades of rejection, although the fact that it is a rather nonspecific marker must be taken into account. Given that some immunosuppressive agents modulate substances that intervene in apoptosis, one explanation for this finding could be that the patients had higher degrees of immunosuppression.
One limitation to our study is the fact that the sample is exploratory and, thus, relatively small in size, although, as we have pointed out, the number of biopsies studied constitutes quite an extensive series. Another limitation is the lack of a confirmatory morphological test, in view of the publication of studies concerning the inaccuracy of TUNEL in the detection of apoptosis and of the possibility that similar DNA fragmentation is produced in other processes involving cell death. Finally, although endomyocardial biopsy continues to be the best method for diagnosing rejection, we should keep in mind its limitations for detecting this condition.
CONCLUSIONS
We have detected the loss of cardiomyocytes due to apoptosis in the transplanted heart. We found no correlation between the apoptosis detected and the grade of acute cellular rejection or a temporal pattern from the time of transplantation. Our data indicate that there could be an inverse correlation between the grade of rejection and the serum TNFα levels. We consider that none of the three apoptosis-related serum parameters that we have studied (soluble Fas, IL-6, and TNFα) is suitable for the noninvasive diagnosis of acute cellular rejection.
ACKNOWLEDGMENTS
The authors thank the Pathology Service of Hospital Puerta de Hierro, Isabel Millán (Biostatistics Section) and Cristina Fernández.
ABBREVIATIONS
IL-6: interleukin 6
ISHLT: International Society for Heart
and Lung Transplantation
TNFα: tumor necrosis factor alpha
TUNEL: TdT-mediated dUTP nick end labeling
Source of funding: Fundación Mapfre Medicina.
Correspondence: Dr. C. Cristóbal.
Servicio de Cardiología. Hospital Universitario de Fuenlabrada. Camino del Molino, 2. 28942 Fuenlabrada. Madrid. Spain
E-mail: ccristobal.hflr@salud.madrid.org
Received June 22, 2009.
Accepted for publication March 3, 2010.