Keywords
INTRODUCTION
Rupture or dissection of the aortic root is the most common cause of death in patients with Marfan syndrome and accounts for the reduced life expectancy (less than 30 years) normally associated with these patients.
Since 1968, when Bentall and de Bono1 described the replacement of the aortic valve and ascending aorta with a tube graft containing a prosthesis, this procedure has traditionally been chosen for the repair of aneurysms affecting the aortic root, generally with excellent results. The intervention always requires replacement of the aortic valve with a prosthesis, even if the valve is morphologically normal, and the use of lifelong anticoagulant therapy if, as is most often the case, a mechanical prosthesis is used. To prevent complications associated with the prosthesis and anticoagulant therapy, new surgical techniques were proposed in the 1990s aimed at conserving the patient's aortic valve. Among those techniques, the best results have been obtained with the David procedure,2 in which the aortic valve from the patient is reimplanted in a tube graft that replaces the diseased aorta.
In the last 2 years, we have used the David procedure with reconstruction of the aortic sinuses in 40 patients. Eighteen of those patients had Marfan syndrome. We describe our experience in this patient group, which we consider to be ideal for this type of intervention, given their age and other factors.
METHODS
Between April 2004 and April 2006 we used the David procedure for valve-sparing aortic root replacement in 40 patients with annuloaortic ectasia or aneurysm of the aortic sinuses (Figure 1). Eighteen had Marfan syndrome, diagnosed according to the Ghent criteria,3 with a median age of 29 years (range, 13-55 years). Transthoracic echocardiography was performed in all patients prior to surgery. If the acoustic window was suboptimal, transesophageal echocardiography was performed. Two-dimensional diastolic measurements were made of the diameter of the annulus, the aortic sinuses (maximum diameter of the aortic root between the annulus and the sinotubular junction), the sinotubular junction, the proximal ascending aorta, and the distal ascending aorta. Color Doppler was used to classify the degree of aortic regurgitation from I to IV. The median diameter of the aortic sinuses was 53 mm (range, 46-59 mm). Twenty-four percent of patients had grade-III aortic regurgitation, 12% grade II, and 64% grade 0/I.
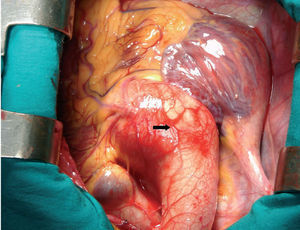
Figure 1. Photograph of the surgical field. Aneurysm of the aortic sinuses measuring 5 cm in a 22-year-old patient with Marfan syndrome.
The rest of the thoracic aorta was studied by magnetic resonance imaging and/or computed tomography, without evidence of dilatation at other sites, except for 1 patient who had previously undergone surgery for a type B dissection and who presented an abdominal aortic aneurism.
Surgical Technique
Surgery was indicated when the diameter of the aorta at the level of the aortic sinuses was greater than 45 mm or when a progressive increase of more than 2 mm per year was documented and when the anatomy of the aortic valve leaflets and the rest of the aortic root were favorable (normal appearance of the leaflets, without prolapse and symmetrical).
In all patients, aortic valve reimplantation was performed using the David procedure.2 Following establishment of extracorporeal circulation and aortic cross-clamping, aortotomy was performed and the aortic valve leaflets carefully inspected along with the rest of the aortic root. If the aortic valve was considered to be conservable, the diseased aorta was resected leaving borders of 2 to 3 mm of the wall of the aorta along with the valve leaflets (Figure 2). To reconstruct the aortic sinuses, in 7 patients we used the Gelweave Valsalva prosthesis described by de Paulis et al.4 This prosthesis is made of Dacron but, unlike the other available prostheses, it includes preformed aortic sinuses (in 6 patients we used a 30-mm prosthesis and in 1 patient a 28-mm prosthesis was used). In another 10 patients we performed the Stanford modification,5 using 2 Hemashield Dacron prostheses (in 7 patients a 34-mm proximal graft was used and in 3 patients a 32-mm graft; the distal graft was 28 mm). After choosing the size of the graft according to the method used by David and Feindel,2 between 9 and 12 mattress sutures (Ethibond 4/0) were placed horizontally below the valve leaflets in the fibrous portion of the aortic annulus and just at the attachment of the leaflet to the annulus in the muscular portion. The graft was tied to stabilize the annulus and the valve leaflets were reimplanted, resuspending the commissures and suturing the remains of the wall of the aorta continuously with 4/0 Prolene (Figure 3). The procedure was completed with the reimplantation of the coronary buttons with 5/0 Prolene using the technique of Bentall and de Bono, and with distal anastomosis of the ascending aorta.
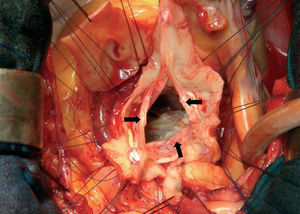
Figure 2. Photograph of the surgical field. Appearance of the aortic valve leaflets with a remnant of the wall of the aorta measuring 2 to 3 mm that will be used to reimplant the valve in the Dacron tube graft. Various mattress sutures are visible that pass below the attachment of the valve leaflets in the sinotubular junction.
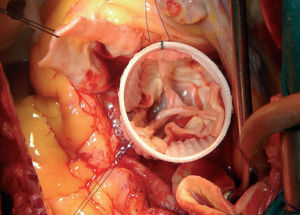
Figure 3. Appearance of the aortic valve leaflets reimplanted in the Gelweave Valsalva prosthesis.
Follow-Up
The median follow-up was 8 months (range, 1-24 months). Transesophageal echocardiography was performed in all patients in the operating theater following reimplantation. An echocardiogram was performed prior to discharge and then clinical follow-up and echocardiography were performed at 1 month. The follow-up protocol included echocardiography at 6 months and then annually in the absence of events. All patients received oral anticoagulation therapy for the first 2 months following surgery, and then subsequently with acetylsalicylic acid (100 mg/d).
RESULTS
The aortic valve could be preserved in 17 patients and displayed correct valve function and normal anatomy of the aortic root in the transesophageal echocardiogram performed during the operation. One patient displayed moderate aortic regurgitation in the operating theater following repair. Consequently, a decision was made to implant a mechanical prosthesis in the graft without it being necessary to repeat the coronary anastomosis. One patient displayed severe mitral regurgitation, a large aneurysm of the interatrial septum, and severe tricuspid regurgitation. In that patient, mitral repair was performed with quadrangular resection of the posterior leaflet and ring annuloplasty, closure of the interatrial septum with a bovine pericardial patch, and tricuspid ring annuloplasty. Normal valve function was observed for all 3 valves in the echocardiogram performed during the operation.
There was no operative or in-hospital mortality. The median length of extracorporeal circulation and aortic cross-clamping was 159 minutes (range, 111-280 minutes) and 140 minutes (range, 104-265 minutes), respectively. In 1 patient, surgery was repeated within a few hours due to bleeding; there were no other noteworthy postoperative complications. The median stay in the postoperative intensive care unit was 2 days (range, 1-4 days) and the median hospital stay was 8 days (range, 6-12 days).
Echocardiography was performed in all patients prior to discharge; 8 patients maintained grade-I aortic regurgitation, 1 patient had grade-II aortic regurgitation, and the remainder did not display aortic regurgitation.
No hemorrhagic or thromboembolic complications were observed during follow-up.
One patient aged 55 years died 9 months after surgery due to rupture of an abdominal aneurysm. The remaining patients were in New York Heart Association functional class I and during follow-up 1 patient exhibited grade-II aortic regurgitation, 8 grade-I regurgitation, and 8 did not have aortic regurgitation.
DISCUSSION
Although replacement of the aortic root using the technique described by Bentall and de Bono with various modifications6 has displayed excellent results and is considered by many surgeons to be the standard against which all other techniques should be compared, late complications due to the use of mechanical prostheses and chronic anticoagulation therapy are common.
Hagl et al7 presented a series of 142 patients, of which 10 had Marfan syndrome, who were surgically treated using the Bentall technique. One patient died in hospital and 2 suffered acute cerebrovascular accidents. The rate of mortality over a mean follow-up period of 3.5 years was 4.2%. Despite these good results, during 8 years of follow-up, 22% of the patients presented events related to the prosthesis and 7% had significant hemorrhagic complications.
Gott et al8,9 reported their experience over 24 years in 271 patients with Marfan syndrome in whom aortic root replacement was performed. The Bentall technique was used in 232 patients. In-hospital mortality was restricted to 2 patients. The survival at 24 years was 83%; 14 patients (6%) had presented embolic events (13 cerebral emboli and 1 peripheral embolus) and 11 patients (5%) had prosthetic valve endocarditis.
Kvidal et al10 undertook long-term follow-up in 424 patients in whom a mechanical aortic valve prosthesis was implanted. In that study, the authors observed an annual incidence of thromboembolic events of 4.4% and an incidence of bleeding associated with anticoagulation of 8.5%.
In fact, complications due to anticoagulation contribute to 75% of the complications associated with mechanical prostheses, and in the literature it has been reported that the annual incidence of thrombotic or hemorrhagic complications ranges from 2% to 4%.11-13
In patients with Marfan syndrome, if the indication for surgery is early, the valve leaflets are usually unaffected. Even in cases in which there is aortic regurgitation, the leaflets generally maintain a normal morphology and the pathophysiologic mechanism responsible for the regurgitation is annuloaortic ectasia or dilatation of the aortic sinuses.14 Preservation of valve leaflets with defective collagen could be an argument against the use of the technique in these patients, but the excellent results of David et al15 and Leyh et al16 with a follow-up of more than 8 years, demonstrate that the main problem in Marfan syndrome is the annuloaortic ectasia at the aortic root rather than structural degeneration of the valve leaflets. In addition, the tissue structure of the aortic valve leaflets is not essentially different from that of the mitral valve leaflets, and mitral valve repair has been shown to be stable over a prolonged follow-up period.8
Early intervention in these patients is the best way to prevent deterioration of the aortic valve, since with aortic sinus diameters of more than 5.5 cm the leaflets appear thin and elongated and the results of repair are more dubious.
Both in the case series of David et al15 and that of Leyh et al,16 the results of aortic valve reimplantation over a follow-up period of 8 years show that more than 90% of patients are free of aortic regurgitation higher than grade II and more than 95% do not require repeat surgery, without any recorded thromboembolic events or bleeding.
In a retrospective study, Kallenbach et al17 compared 78 cases of aortic valve reimplantation with 269 patients in whom the Bentall technique had been employed; those authors observed similar results with the exception of thromboembolic events and bleeding, which occurred in 12% of patients in whom a composite, valve-containing graft was implanted.
At the time of writing, the largest case series reported in Spain is that of Nistal,18 in which 33 patients were treated between 1994 and 2003 with valve reimplantation using the David procedure. Three of those patients had Marfan syndrome. With a follow-up of 9 years, the probability of remaining free of aortic insufficiency at grade III or above was 81% (9)% and 90% (7)% did not require valve replacement.
Encouraged by those data, we began to use the technique in April 2004 and at the time of writing we have used the technique in 40 patients, 18 of whom had Marfan syndrome. With a mean follow-up of 7 months, 16 patients are free of aortic regurgitation greater than grade I and 1 patient continues to exhibit grade-II aortic regurgitation.
Although the results reported by David or Haverich are excellent with the use of a Dacron graft, we reconstructed the aortic sinuses because we considered it to contribute to improved durability of the valve leaflets.
Preservation of the native aortic valve appears to be especially indicated in Marfan syndrome, if we take into account that surgery is proposed at a young age and that many patients require other interventions due to collagen abnormalities (ocular, musculoskeletal, etc). In those patients, the lack of requirement for chronic anticoagulant therapy removes a serious long-term risk factor.
The possible disadvantages of using this technique include the current lack of information regarding the long-term durability of the aortic valve and the greater complexity compared with the Bentall technique, necessitating more demanding training. It also requires a more prolonged period of aortic cross-clamping, although with the current methods for myocardial preservation this does not translate into a higher incidence of low cardiac output or perioperative infarction.
CONCLUSIONS
Preservation of the native aortic valve by valve reimplantation is a technically feasible procedure and has shown excellent medium-term results, since it avoids the complications associated with mechanical prostheses and chronic anticoagulation. If the reimplanted aortic valve maintains adequate long-term functionality, this will become the technique of choice for surgical treatment of ascending aortic aneurysm in patients with Marfan syndrome.
In the meantime, while it would be appropriate for surgeons to be familiar with both techniques, in the event of doubt or lack of training, it is preferable to use the procedure of Bentall and de Bono, which is less technically demanding and has proven to be reproducible and have a more rapid learning curve.
See editorial on pages 461-3
Correspondence: Dr. A. Forteza.
Servicio de Cirugía Cardiaca. Hospital Universitario 12 de Octubre.
Agastia, 45, 3.o C. 28027 Madrid. España.
E-mail: aforteza.hdoc@salud.madrid.org
Received December 16, 2005.
Accepted for publication November 23, 2006.