Keywords
INTRODUCTION
The main purpose of treatment for acute myocardial infarction (AMI) is to reestablish tissue perfusion.1 Even in patients with a patent culprit artery, a microvascular perfusion defect is associated with a poorer prognosis.1-5
The extent of the perfusion defect may vary in the months following an AMI.5,6 As measured by cardiac magnetic resonance (CMR), the evolution of perfusion is controversial. Several authors have defined abnormal perfusion as a phenomenon that is always transient in patients with TIMI 3 flow, mainly on the basis of analyses of delayed gadolinium enhancement sequences.7,8 However, first-pass imaging studies have found that perfusion abnormalities can become chronic.9,10 At the threshold of the era of regenerative medicine,11 it is important to clarify whether or not myocardial perfusion alterations can persist in the chronic phase after an AMI with maintained TIMI 3 flow.
The ideal method to analyze myocardial perfusion is still not clear.1 Although it is limited by its invasive nature, intracoronary myocardial contrast echocardiography (MCE) allows clear assessment of perfusion and may be a reference technique.2,5,12
In this study, we used MCE to assess the presence of microcirculation alterations in the chronic phase after AMI, as well as its significance in relation to ventricular dilation and systolic function.
METHODS
Study Group
We prospectively included 67 consecutive patients with a first AMI and ST segment elevation treated with thrombolysis within the first 6 h after onset of pain and who underwent cardiac catheterization (with angioplasty, if necessary) before discharge. The inclusion criteria were: a) stable clinical course with no complications in the first 6 months; b) single-vessel disease with TIMI 3 flow and residual stenosis <50% in the culprit artery at the end of predischarge catheterization and in the sixth month; and c) absence of contraindications for CMR. We excluded 20 patients with multivessel disease (10 cases), TIMI flow <3 (2 cases), restenosis (5 cases), claustrophobia (2 cases), and reinfarction (1 case), giving a final study group composed of 47 patients.
The study met the requirements of the Declaration of Helsinki and was approved by the Ethics Committee of our institution. All subjects signed the informed consent to participate.
The patients included in the study group are part of a prospective protocol that assesses various aspects of remodeling, perfusion, and myocardial viability by different invasive and noninvasive techniques.5,9,12,13 We recently analyzed the evolution of microcirculation using first-pass gadolinium imaging with CMR in 40 patients from this series.9 The 17 patients included in this protocol underwent a preliminary analysis of perfusion evolution with MCE, angiography and CMR.5 In this paper, we present a more comprehensive analysis of the evolution of perfusion abnormalities by MCE in a group of 47 patients.
Cardiac Catheterization
Cardiac catheterization was performed 4 (±1) days after the AMI. Salvage angioplasty was done within the first 12 h after thrombolysis in 3 patients for chest pain and persistent ST segment elevation. Catheterization was repeated in these 3 patients before discharge and the perfusion indexes (both TIMI flow and MCE) were determined at that time.
The culprit artery was the anterior descending artery in 29 cases and the right coronary in 18 cases. An intracoronary stent was implanted in 41 patients (87%) with residual stenosis >50%. At completion of predischarge catheterization, all patients had TIMI 3 flow and residual stenosis <50%. Cardiac catheterization was repeated at 179 (±8) days after AMI, and TIMI 3 flow and residual stenosis <50% were confirmed in all patients included in the study.
Intracoronary Myocardial Contrast Echocardiography
At the end of cardiac catheterization (both predischarge and at six months), MCE was performed according to the previously established protocol in our laboratory.5 A 1-mL bolus (concentration, 350 mg/mL) of Levovist (Schering AG, Germany) was injected, followed by 5 mL of saline solution through the catheter that revascularized the culprit artery for the parasternal and apical views. Successive 1-mL contrast boluses were administered if necessary. There were no adverse effects in more than 100 studies. The images were recorded in real time using second harmonic technology with a VingMed 750 echocardiograph (General Electrics, USA); gain and compression were optimized in each case and did not vary during the studies.
Analysis of Intracoronary Myocardial Contrast Echocardiography
The images were digitized and analyzed on separate days by an experienced observer who was unaware of the results obtained in the other examinations. Images were taken with maximum contrast intensity in end-systole, and the area at risk was defined as the region including segments dependent on the culprit artery in the 17-segment model.14 Quantitation of perfusion was done using MATLAB 6.5 (The Mathworks Inc., USA).12 A region of interest was defined in each field; the baseline intensity (video intensity units) was subtracted and standardized by the intensity of the segment that showed normal contractility and maximum intensity (video intensity units), yielding in all cases a standardized perfusion range of 0 to 1. Based on our experience, we defined abnormal perfusion to exist in a segment if its standardized range was <0.75.12 A patient was considered to have poor perfusion in the infarct area if more than one segment showed abnormal perfusion.
The intraobserver variability with regard to presence or absence of normal perfusion was analyzed in 15 patients; these studies were assessed twice at least 3 months apart by the observer who quantitated all the data. When using the methodology described, the variability was 0%.
Cardiac Magnetic Resonance
Cardiac magnetic resonance (1.5-Tesla, Sonata Magnetom, Siemens, Germany) was performed 7 (±1) days (at least 48 h after catheterization) and 184 (±11) days after the AMI, following the protocol of our laboratory.9,13 Electrocardiogram-gated images were recorded during breath-holding. The cine-MRI images (true fast imaging with steady state precession sequences, repetition time/echo time: 3.2/1.6 mso angle, 61o; matrix; 256x128; slice thickness, 6 mm) were acquired in 2-, 3, and 4-chamber views and every 1 cm in the short-axis views.
Following administration of 0.1 mmol/kg of gadolinium-diethylenetriaminepentaacetic acid contrast (Magnograf, Juste SAQF, Spain) at a flow rate of 3 mL/s, the delayed gadolinium enhancement images were taken 10 min after contrast injection using a true fast imaging with steady state precession (repetition time/echo time: 2.5/1.1 ms; slice thickness, 6 mm; angle, 50o; matrix, 195x192), canceling the myocardial signal.
Cardiac Magnetic Resonance Analysis
The studies were analyzed by an experienced observer using QMASS MR 6.1.5 (Medis, The Netherlands). The end-diastolic and end-systolic volumes (mL/m2), ventricular mass (g/m2), and ejection fraction (%) were quantitated by planimetry of the cine images.
In the delayed uptake images, late enhancement was considered to exist if the signal intensity was >2 standard deviations with respect to a remote, noninfarcted area9; the percentage of ventricular mass was calculated with delayed enhancement (percentage of infarction/infarcted mass). All indices were reevaluated at six months.
Intraobserver variability was analyzed in 15 patients; the CMR studies were assessed twice by the observer, who quantitated all the data at least 3 months apart. The intraobserver variability was 4.1 mL/m2 (±2.9) mL/m2 for end-diastolic volume, 2 mL/m2 (±1.4) mL/m2 for end-systolic volume, and 2.4% (±2.4%) for ejection fraction.
Statistical Analysis
Continuous variables are expressed as mean (± standard deviation) and qualitative variables, as percentages. For the continuous variables, the 2 groups were compared by Student t -test for paired and unpaired data. Comparisons between more than 2 groups were done using analysis of variance (with the Bonferroni test to analyze the differences between subgroups). The χ2 test was used to compare percentages. A P value less than < .05 was considered statistically significant. SPSS 11.0 (SPSS Inc., USA) was used for the statistical analysis.
RESULTS
The baseline characteristics of the 47 patients are shown in Table 1.
Abnormal Perfusion on Intracoronary Myocardial Contrast Echocardiography
Abnormal perfusion was detected by MCE in 20 patients (43%) at one week and 10 (21%) at six months. Abnormal perfusion was related to higher ventricular volumes, more severely depressed ejection fraction, and higher percentage of infarct mass, both at one week and at six months. Only 1 case of abnormal perfusion at six months was associated with a larger ventricular mass in the chronic phase (Tables 2 and 3).
Regarding the evolution of the end-diastolic volume from one week to the six months, no significant increases were detected in the overall study group (70 [±21] vs 69 [±24] mL/m2; P=.9) or in patients with normal (65 [±14] vs 64 [±17] mL/m2; P=.8) or abnormal perfusion (78 [±26] vs 77 [±32] mL/m2; P=.9) at one week. Following a separate analysis of the 20 patients with abnormal perfusion in the first week, those in whom perfusion was normal at the six months (n=10) showed a trend toward decreases in the end-diastolic volume (69 [±15] vs 64 [±12] mL/m2; P=.2), whereas those who presented chronic abnormal perfusion showed a trend toward increased end-diastolic volume (82 [±33] vs 91 [±41] mL/m2; P=.1).
Evolution of Perfusion From One Week to Six Months
The number of segments per patient with abnormal perfusion as determined by MCE decreased from one week to six months (2 [±2.6] vs 0.8 [±1.8] segments; P<.0001).
The 27 patients with normal perfusion on MCE in the one week maintained this condition in six months. Among the 20 patients with abnormal perfusion at one week, 10 (50%) achieved normal perfusion in the six months, whereas poor perfusion became chronic in 10 patients (21% of the entire group) (Figures 1 and 2).
Figure 1. Evolution of perfusion on intracoronary myocardial contrast echocardiography. All patients with normal perfusion at one week maintained this condition at six months. Abnormal perfusion persisted in at six months in 10 of the 20 patients who had abnormal perfusion at one week.
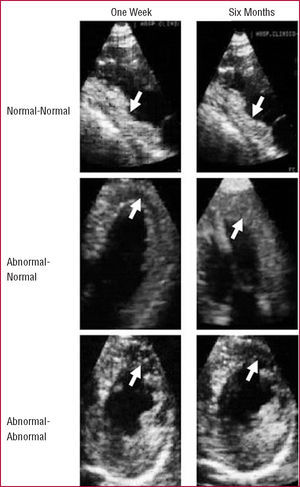
Figure 2. Intracoronary myocardial contrast echocardiography. Top: a patient with an inferior infarction and normal perfusion in the infarct area (arrows), both at one week (left) and the six months (right). Middle: a patient with an anterior infarction and abnormal perfusion in the infarct area (arrows) at one week (left) and normal perfusion in the six months (right). Bottom: a patient with an anterior infarction and abnormal perfusion in the infarct area (arrows) at both one week (left) and the sixth month (right).
Patients with chronic abnormal perfusion exhibited larger ventricular volumes and masses and a more severely depressed ejection fraction at 6 months than the other 2 subgroups. Furthermore, they presented a greater percentage of infarct mass than patients with normal perfusion from the start, but no differences with respect to the subgroup with late normalization of perfusion (Figure 3).
Figure 3. Based on the evolution of perfusion on myocardial contrast echocardiography from onet week to six months, the patients were divided into 3 groups: normal-normal (n=27), abnormal-normal (n=10), and abnormal-abnormal (n=10). Patients with sustained abnormal perfusion showed a more severely depressed ejection fraction, greater percentage of infarct mass (top), and larger ventricular volumes and mass (bottom) in the chronic phase. EDV indicates, end-diastolic volume; EF, indicates ejection fraction; ESV, end-systolic volume. aP<.01 for abnormal-abnormal vs the other 2 groups. bP<.01 for abnormal-abnormal vs normal-normal; P=nonsignificant for abnormal-abnormal vs abnormal-normal.
DISCUSSION
In a group of patients with a first AMI, single-vessel disease, and maintained TIMI 3 flow, MCE showed that perfusion alterations can persist during the chronic phase and are related with poorer ventricular function parameters.
Evolution of Perfusion on Cardiac Magnetic Resonance
Analysis of the evolution of perfusion abnormalities as measured by CMR has provided uneven results. Wu et al8 and Hombach et al7 observed acute phase perfusion defects in 25% and 46% of their patients, respectively. In both studies, the abnormalities disappeared in the chronic phase in all cases.
This transient alteration is apparently more common in studies that assess microcirculation on delayed enhancement images (contrast defects surrounded by necrotic tissue with late enhancement). Studies analyzing perfusion on first-pass gadolinium images have indicated that microcirculation alterations can become chronic in some cases. Taylor et al10 observed this phenomenon in a group of 16 patients.
In 40 patients in the study group of the present series, we recently observed that chronic perfusion abnormalities can affect up to 14% of segments in the infarct region in the postinfarction chronic phase by an analysis of first-pass gadolinium images.9
At the threshold of the era of regenerative medicine,11,15 this concept requires clarification. Patients with poor microvascular perfusion have a poorer prognosis,1-5 and the possibility of chronic microcirculation defects should not be ruled out, since these cases could be candidates for regenerative medicine in the future.
Abnormal Perfusion on Intracoronary Myocardial Contrast Echocardiography
The kinetics of gadolinium must be taken into account when analyzing the evolution of perfusion abnormalities with CMR16: it is an extracellular agent with a high capacity for diffusion in the interstitial space. Failure of gadolinium to fill the interstitium in the acute phase could be due not only to microvascular damage, but also to massive interstitial edema in extensive infarctions that could impede the entry of gadolinium. Once the inflammatory response disappears, the contrast again occupies the interstitium, which would explain the disappearance of microvascular alteration in all cases in the chronic phase.
In view of these uncertainties, we decided to assess microcirculation using an alternative method. Because of the purely intravascular character of echocardiography contrast, MCE allows direct assessment of coronary microcirculation rather than interstitium. The contrast was injected directly into the coronary artery and reached the small vessels.2,5,12 The large number of bubbles allows excellent definition of the perfusion (Figure 2).
This methodology was able to confirm the trend toward spontaneous improvement of microcirculation observed with different techniques.6-8 Half the patients with abnormal perfusion on MCE study achieved normal perfusion in the chronic phase. The most interesting finding was that MCE allowed detection of a subgroup (21% of the total) in which abnormal perfusion became a chronic phenomenon.
A persistent perfusion abnormality was associated with more highly depressed systolic function and greater dilation of ventricular volumes in comparison with normal perfusion, whether from the first week or for late normalization. In fact, the ventricular mass was only larger in cases of chronic perfusion alteration. As expected, the percentage of infarct mass was only lower in the subgroup with normal perfusion from the start. Lastly, the only subgroup that showed a trend (nonsignificant, probably because of the small number of patients, n=10) toward progressive ventricular enlargement were those with a chronic perfusion abnormality. These data indicate that abnormal perfusion in the chronic phase is not merely an incidental finding, but has important consequences for ventricular function.
Limitations
For the purpose of carrying out a careful assessment of the objectives (basically pathophysiological), we enrolled a carefully selected group of patients. Therefore, the results are only applicable to series similar to the one presented. We used intracoronary MCE which is an invasive procedure; however, intravenous injection has shown similar results.12 In our experience, MCE with intracoronary injection allows a more adequate analysis of perfusion and therefore, we chose this method to ensure the objectives set out. The MCE studies had to be done upon completion of catheterization; this could correlate to a higher percentage of cases with abnormal perfusion in comparison with later analyses.
CONCLUSIONS
Most patients with sustained TIMI 3 flow present normal myocardial perfusion, whether from the acute phase or by late spontaneous normalization with no need for further interventions. In a small group of patients, abnormal perfusion persists despite a patent artery. These patients show greater deterioration of left ventricular function and in the future, could be candidates for regenerative medicine.
See editorial on pages 468-70
This study was funded by a grant from Guidant.
ABBREVIATIONS
AMI: acute myocardial infarction
CMR: cardiac magnetic resonance
MCE: myocardial contrast echocardiography
Correspondence: Dr. V. Bodí.
Servicio de Cardiología. Hospital Clínico y Universitario de Valencia.
Blasco Ibáñez, 17. 46010 Valencia. España.
E-mail: vicentbodi@hotmail.com
Received July 6, 2006.
Accepted for publication December 12, 2006.