Keywords
INTRODUCTION
Ablation of atrial fibrillation (AF) during cardiac surgery is becoming increasingly common. Sources of energy for surgical ablation include radiofrequency (RF) discharges, microwaves and cryoablation. Ultrasound waves and lasers may also be used in the near future. Ablation is a quick and simple procedure that can treat AF by electrically isolating atrial regions.1-6
Atrial fibrillation is of particular interest to surgeons because it is common in preoperative patients--prevalence is as high as 16% in Spain even if we only include the permanent form of AF. Prevalence is particularly high in patients with mitral valve disease--as many as 60% suffer from permanent AF7 compared to 0.6% of the general population.8 A recent study has reported that AF doubles the risk of mortality.9 The clinical repercussion of AF is probably greatest in patients who are indicated for cardiac surgery and, moreover, the ablation technique is probably easiest during a surgical procedure.
In the present study, we report our experience with 93 patients in whom cardiac surgery was associated with ablation of permanent AF.
PATIENTS AND METHODS
Between June 2000 and June 2003, 93 patients with heart disease requiring surgery and permanent AF for more than 3 months underwent ablation of the arrhythmia during cardiac surgery. All patients were informed of the procedure and gave their informed written consent. Patient selection was not arbitrary. This depended on patients' age, preoperative health status and the surgeon's criteria. Initially, there were no exclusion criteria, but later we excluded patients with a presurgical assessment indicating high risk and those with atrial calcification. The study group was formed of 31 men and 62 women aged between 39 years and 78 years (mean, 61±9 years). The preoperative duration of AF was documented by electrocardiography and ranged from 3 months to 24 years (mean, 6.0±5.7 years). Table 1 shows the main clinical and echocardiographic characteristics of patients and their arrhythmias. At the time of the intervention, all patients were receiving some type of antiarrhythmic agent, usually digoxin or amiodarone. Twenty patients (21.5%) had a history of thromboembolism. Most of the patients (80.7%) presented with left atrial (LA) dilatation, defined as an anteroposterior atrial diameter greater than 45 mm.
The reasons for heart surgery varied, though the most common was mitral valve disease. The type of primary heart disease and the corresponding surgical procedures are shown in Table 2. The preoperative score to assess surgical risk in this group of patients was 5.2±2.8 according to the EuroSCORE predictive model10 and 5.8±2.3 according to the model of St. Luke's Medical Center (Chicago, United States of America).11 These scales suggested that the risk of hospital mortality for this group of patients was intermediate, which corresponded to 5.9% for the EuroSCORE model and 6.4% for the model of St. Luke's Medical Center.
Surgical Ablation Protocol
Compartmentalization of both atria and isolation of pulmonary veins were carried out by ablation lines using the lesion set described by Cox12 for the maze intervention. The same lesion sets as those described previously in this journal were used for all patients.13 Briefly, the ablation procedure began in the right atrium (RA) without extracorporeal circulation (ECC). Two separate lines were ablated on the epicardium, the first along the crista terminalis from the superior to inferior vena cava, including the cavotricuspid isthmus, and the second, perpendicular to the first from the inferior right pulmonary vein to the tricuspid annulus along the right appendage. Next, the heart-lung apparatus was connected and ECC was initiated. Systemic hypothermia of 30ºC was induced. LA ablation started from the endocardium under moderate hypothermia. The ostia of the left and right pulmonary veins were independently isolated by 2 circumferential lesions connected to one another by a new line along the posterior atrial wall. Two further ablation lines connected the circumference of the left pulmonary veins with the mitral annulus and the left appendage. Once ablation was finished, the cardiac surgery was performed.
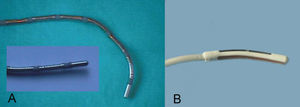
A unipolar RF energy source was used in the first 85 patients, whereas microwave energy was used in the last 8 patients. Radiofrequency energy was applied with a ThermaLine® (Boston Scientific Corporation, Natick, MA) surgical probe with a malleable shaft, at a power of 100 W for 120 seconds and a temperature set-point of 85ºC (Figure 1). Microwave energy was applied with the Flex 4® (AFx Inc., Fremont, CA) malleable surgical probe at a power of 65 W for 65 seconds for the epicardial lesions and 65 W for 45 seconds for the endocardial lesions.
Fig. 1. Surgical ablation catheters. A: unipolar multielectrode surgical probe with a malleable shaft, with 7 10 mm coil electrodes with 3 mm spacing. B: continuous microwave probe with 4 mm antenna.
Pre and Postoperative Protocol and Management
Administration of the patients' normal antiarrhythmic drugs was not discontinued before the intervention. Intravenous amiodarone treatment was started during the operation and continued at 1200 mg/day for the first 48 hours, followed by oral doses of 200 mg/day of the same drug for the first 3 months after the operation. Patients in stable sinus rhythm then stopped taking the drug. If amiodarone was contraindicated, sotalol was administered orally (80 to 160 mg/day). The anticoagulants were discontinued after 3 months in patients with no mechanical heart prothesis and with effective atrial contraction according to the echocardiographic examination. Atrial contraction was considered effective when an A-wave was present with an A/E ratio of >0.25 (where A corresponds to peak A-wave velocity and E to peak E-wave velocity).
Episodes of postoperative AF/flutter during follow-up were treated with external electrical cardioversion. A maximum of 3 applications per patient were allowed after hospital discharge. If the patient experienced further recurrences, the surgical procedure was considered an ineffective treatment of the arrhythmia and other alternatives were sought.
The preoperative and postoperative echocardiographic examinations measured atrial diameter in millimeters in addition to the normal echocardiographic parameters. The atrial volume was calculated from measurement of the diameters along the 3 perpendicular axes assuming an elliptical shape.14 The atrial contribution to ventricular filling was assessed by Doppler echocardiography, measuring the peak velocity of atrial contraction (A-wave) in m/s and the A/E ratio. The follow-up electrocardiographic and echocardiographic examinations were performed on hospital discharge, after 3 and 6 months, and then every year. Holter monitoring was only used when the patient reported new episodes of palpitations or symptoms suggestive of chronotropic insufficiency.
The data are expressed as mean ± standard deviation or as frequency (percentage). The corrected χ² test was used to analyze the variables related to postoperative recurrence of AF, whereas the Fisher exact test was applied for categorical variables. Ordinal variables were compared using the Student t test for normal distributions and the Mann-Whitney test for non-normal distributions. The AF/flutter free survival curve was calculated using the Kaplan-Meier method. The mean duration of follow-up in this study was 292 days (range: 31-1036 days). Eighty-two patients (88.1%) had a follow-up longer than 6 months; mean follow-up for these patients was 332 days (range: 174-1036 days). Statistical significance was set at P<.05.
RESULTS
According to our experience, ablation of permanent AF during cardiac surgery has a success rate of 83.8%. Analysis of procedural success by period showed that the incidence of sinus rhythm and arrhythmias was variable. During the postoperative hospital stay, 74.1% of the patients presented some type of supraventricular arrhythmia, 2 patients (2.1%) had sinus node dysfunction, 6 patients (6.4%) had nodal rhythm for more than 7 days, 18 patients (19.3%) had first-degree atrioventricular block, 1 patient had type-1 second-degree atrioventricular block, 4 patients (4.2%) had third-degree atrioventricular block (2 patients with atrioventricular canal defect), 4 patients (4.2%) had paroxysmal atrial flutter, 6 patients (6.4%) had chronic AF, 7 patients (7.5%) had paroxysmal episodes of AF, and 37 patients (39.4%) had persistent AF recurrence. One patient had a permanent pacemaker implanted before hospital discharge. With the patients who died during the postoperative period in hospital excluded, 64 patients (72.7%) had recovered sinus rhythm on discharge, 1 patient maintained atrial flutter, and 23 patients (26.1%) had persistent AF.
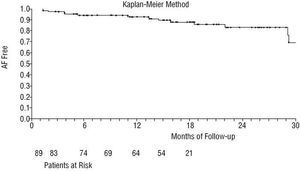
During the follow-up period following hospital discharge, 6 patients (6.8%) presented sinus node dysfunction. Of these, 3 had received a permanent pacemaker. Six patients (6.8%) had to be re-hospitalized due to recurrence of AF/flutter, 5 because of paroxysmal episodes of AF/flutter and 1 patient because of supraventricular paroxysmal tachycardias. Of the 82 patients who were followed up for more than 6 months, 84.1% recovered sinus rhythm. Currently, 16.1% still have permanent AF/flutter and 83.8% have recovered and maintained sinus rhythm. Figure 2 shows the survival curve for postoperative AF/flutter free survival.
Fig. 2. Survival curve for postoperative recovery of sinus rhythm. AF indicates atrial fibrillation.
In the univariate statistical analysis, the factors associated with the failure of the antiarrhythmic procedure were duration of the arrhythmia (P<.02) and the preoperative LA volumes (P<.009). Sex, age, amplitude of the f-wave in the electrocardiogram, type of valve disease and type of energy applied were not related to failure of the technique. Likewise, RA volume did not influence success, which brings into question whether right atrial lesion sets are needed (Table 3).
Three patients (3.2%) had major complications that could, in our opinion, be attributed to the ablation procedure--all of these occurred with RF energy application. One 47-year-old patient with mitral valve replacement suffered cardiogenic shock with severe hypokinesia of the lateral wall in the postoperative echocardiogram on arrival at the intensive care unit. She required immediate balloon counterpulsation and had to undergo further surgery for saphenous vein bypass to the circumflex artery. The cardiogenic shock might have been caused by a coronary vasospasm due to ablation. However, the subsequent coronary angiography allowed us to discard lesions in the coronary tree, including the circumflex artery. A 73-year-old patient with mitral valve replacement and 3 bypass operations died 19 days after the procedure. He was re-hospitalized with acute sepsis associated with repetitive episodes of cerebral ischemia, clinically similar to endocarditis but with a normal transesophageal echocardiogram. Computed brain tomography showed multiple gas embolisms that were hard to interpret given their large size. No postmortem examination findings are available, but we think the underlying cause could have been the development of a fistula between the LA and the esophagus, as described by other authors during similar surgery.15 One 69-year-old patient with mitral valve replacement needed to undergo further surgery 3 months after the initial procedure because of a severe perivalvular leak. We attribute this complication to the use of RF because the echocardiogram was normal 5 days after the procedure and because the leak was located at a point on the RF line that joined the mitral annulus with the left pulmonary veins.
In-hospital mortality was 5.3%--similar to the value expected from predictive models. The causes of death were sudden death on Day 23 after the procedure in a patient with mitral valve replacement, respiratory distress syndrome in a 56-year-old patient with complete atrioventricular channel and severe pulmonary hypertension, and multiorgan failure in 3 patients with prolonged postoperative intubation. The postoperative in-hospital complications varied. One patient presented with a transient cerebral ischemic accident on Day 3 after the procedure, 6 patients required further surgery due to hemorrhage, and 2 patients needed intraaortic balloon counterpulsation support. The mean stay in hospital was 13.8±8.5 days (range: 6-65 days). Although there was no control group, the hospital stay was often extended several days until stable heart rhythm was obtained or, at times, to perform electrical cardioversion before discharge. Three patients (3.2%) died during follow-up after hospital discharge: one patient with atrioventricular canal defect, probably related to complete transient block noted immediately after the procedure; one with aortic valve replacement because of brain hemorrhage related to treatment with acenocoumarol, and 1 patient was thought to have died of late atrial perforation (atrioesophageal fistula). The overall mortality at the end of the study, after an average follow-up of approximately 10 months, was 8.6%. In principle, we only attribute the death of the patient with atrioesophageal fistula to the surgical technique of AF.
We could not find visual evidence for perforation of the LA during the surgical application of endocardial RF. In contrast, 3 point perforations occurred during epicardial ablation with RF of the RA, attributable to excess energy. In 14% of the applications with multielectrode RF application, the ablation lines had gaps of healthy tissue--that is, no decoloration associated with white coagulation tissue due to RF application was apparent. Ablation of such areas was repeated. Ablation with RF was often ineffective in areas of fatty tissue or epicardial fibrosis due to reintervention. In all patients, the RA lesions could be made without ECC.
Effective atrial contractile function, as documented by echocardiography, was reestablished in 50% of the patients. Biatrial contraction was observed in 34 of the 64 patients in sinus rhythm at the time of hospital discharge (53.1%) with a mean mitral A-wave velocity of 0.385±0.379 m/s and a mean mitral A/E ratio of 0.482±0.369. Of the 74 patients without AF at the end of follow-up, 37 (50%) had biatrial contraction with a mean mitral A-wave velocity of 0.580±0.501 m/s and a mitral A/E ratio of 0.466±0.543; 20 (27%) had RA contraction only with a tricuspid A-wave velocity of 0.576±0.596 m/s and a tricuspid A/E ratio of 0.588±0.453; and 17 patients (22.9%) lacked contraction in either atrium. All patients with LA contraction also maintained RA contraction. Although the echocardiographic assessment of systolic atrial contraction is subject to the variability of ventricular preload and afterload, only a slight improvement in the mitral and tricuspid A/E ratio was observed--the differences were not statistically significant.
DISCUSSION
The aim of this study is to report our experience and findings in intraoperative ablation of permanent AF in patients undergoing heart surgery. Using a biatrial lesion set, the permanent AF was treated very effectively--83.8% of the patients recovered sinus rhythm. Mortality during surgery in this group of patients was similar to that expected with predictive surgical models and corresponded to that of the heart disease itself. Therefore, we consider intraoperative ablation as an associated procedure that does not increase in-hospital mortality. Nevertheless, major complications are associated with the ablation procedure--a rate of around 3%--which represents an important degree of surgical morbidity. This forces us to reconsider our protocols and the types of energy source. The complications reported in our study were similar to those described by other groups.15,16 It is difficult to predict which patients are at a higher risk due to ablation during cardiac surgery because of the anatomical variability of patients, both in atrial wall thicknesses and in the conductive properties of the tissue.
The intraoperative ablation systems currently available have been shown to be easily applied and have a high therapeutic effectiveness, but this effectiveness is lower than that obtained from classic maze procedures based on cutting and suturing atrial tissue.2-6,15,17-21 In recent editorials, Dr. James L. Cox,22 one of the pioneers of AF surgery, warned of these poorer outcomes and attributes them to the lesions not being fully transmural. He suggests that cryoablation should be the preferred intraoperative energy source because it ensures a high degree of transmurality and does not damage neighboring structures. At present, no comparative studies of different energy sources are available for ablation during cardiac surgery, though according to the experience of several surgery groups23,24 and our own experience, RF ablation does not usually cause macroscopic lesions in fatty regions or with epicardial fibrosis. Santiago et al25 have described a lower RF transmurality in atria of patients with mitral valve disease compared with healthy tissue. The authors attributed this to structural and conductive differences of the atrial wall itself.
The incidence of AF/flutter immediately after the operation was 45.8%--similar to the incidence described in the literature.4,15,26 The patients experienced a phase of postoperative electrical instability in the first 3 months after the operation before settling on the final rhythm resulting from the ablation procedure.3,4,21 After the first 3 months, around 80% of the patients recovered sinus rhythm.3,4,15,18,27 Different factors seem to be implicated in the etiopathogenic mechanisms of postoperative recurrence of AF, for example, failure to produce transmural lesions or discontinuous ablation lines,24,26 inflammatory processes of surgical trauma (atriotomies, postischemic tissue edema, pericarditis, etc) or greater postoperative adrenergic tone. Given that the incidence of postoperative AF in isolated myocardial revascularization is between 25% and 40%,28,29 it was difficult to determine the percentage of our patients with recurrence of postoperative AF-flutter due to primary failure of the ablation technique. Currently, a percutaneous postoperative procedure can be considered to close possible gaps in the surgical ablation lines, as described by several authors in patients in whom the surgical ablation procedure is ineffective after the first 3 months.30
Contraction of both atria was effectively recovered (A/E ratio >0.25) in 50% of the patients with sinus rhythm in this study. Ablation lines have a harmful effect on atrial contraction.3,31 Melo et al3 report a 42% recovery of right atrial contractility and a 30% recovery of left atrial contractility in 25 patients with mitral surgery and left atrial maze intervention with RF after 6 months of follow-up. Other studies have published better results, with an echocardiographic recovery of 65%2 and 100%,32 although with less strict criteria for effective contraction.
This study has several limitations, mainly derived from the nonstandard sample selection, composed of patients with heart disease requiring surgery and permanent AF. The study included patients with different types of heart disease, although most had mitral valve disease. We did not carry out histologic studies with which to compare macroscopic surgical observations or a postoperative electrophysiological study to demonstrate the effectiveness of the procedure. The assessment of the effectiveness of the procedure is essentially clinical--the Holter study was only carried out in patients with symptoms. The antiarrhythmic treatment during the first months is another important limitation of the findings of this study, particularly because of the short period of follow-up in a type of surgery that requires long-term study.
Our initial experience with surgical ablation of permanent AF to electrically isolate the 2 atria showed an effectiveness of 83.8% without increasing the risk of the primary surgical procedure. The ablation procedure accounted for 3.2% of the major complications. Right atrial epicardial ablation can be performed in all patients without ECC. Although only 26.8% of the patients had recurrence of AF after hospital discharge, postoperative arrhythmias--occurring in 74.1% of the patients--were a major problem. This surgery does not offer immediate results and requires close patient monitoring during the first months, though most patients do eventually attain sinus rhythm.
Correspondence: Dr. F. Hornero Sos.
Servicio de Cirugía Cardíaca.
Hospital General Universitario de Valencia.
Avda. Tres Cruces, s/n. 46014 Valencia. España.
E-mail: hornero_fer@gva.es