Although multiple studies suggest that chronic Chagas cardiomyopathy (CCC) has higher mortality than other cardiomyopathies, the absence of meta-analyses supporting this perspective limits the possibility of generating robust conclusions. The aim of this study was to systematically evaluate the current evidence on mortality risk in CCC compared with that of other cardiomyopathies.
MethodsPubMed/Medline and EMBASE were searched for studies comparing mortality risk between patients with CCC and those with other cardiomyopathies, including in the latter nonischemic cardiomyopathy (NICM), ischemic cardiomyopathy, and non-Chagas cardiomyopathy (nonCC). A random-effects meta-analysis was performed to combine the effects of the evaluated studies.
ResultsA total of 37 studies evaluating 17 949 patients were included. Patients with CCC had a significantly higher mortality risk compared with patients with NICM (HR, 2.04; 95%CI, 1.60-2.60; I2, 47%; 8 studies) and non-CC (HR, 2.26; 95%CI, 1.65-3.10; I2, 71%; 11 studies), while no significant association was observed compared with patients with ischemic cardiomyopathy (HR, 1.72; 95%CI, 0.80-3.66; I2, 69%; 4 studies) in the adjusted-measures meta-analysis.
ConclusionsPatients with CCC have an almost 2-fold increased mortality risk compared with individuals with heart failure secondary to other etiologies. This finding highlights the need for effective public policies and targeted research initiatives to optimally address the challenges of CCC.
Keywords
Abreviations
Chagas disease, caused by the parasite Trypanosoma cruzi, currently affects 6 to 8 million people, primarily in endemic areas in Latin America.1 However, migration has turned Chagas disease into a worldwide public health issue, with nearly 300 000 cases estimated in the USA and 50 000 in Europe.1 During the course of the disease, 30% of the patients will develop chronic Chagas cardiomyopathy (CCC), characterized by rapid heart failure (HF) progression and a high incidence of stroke and fatal ventricular arrhythmias.2 Although the prognosis of HF patients ha significantly improved with the advent of neurohormonal blockade therapies during the last 4 decades, multiple studies indicate a persistent higher risk of adverse cardiovascular outcomes in the CCC population than in other cardiomyopathies (OC).3 Nonetheless, evidence supporting this association is mainly derived from individual original studies and reviews not focused on HF patients, leaving a substantial knowledge gap in this area.4,5 Therefore, this study aimed to systematically assess the evidence comparing the mortality risk in CCC with that of OC.
METHODSData sources and search strategyThis study was performed in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (table 1 of the supplementary data). We searched the PubMed/Medline (National Library of Medicine, United States) and EMBASE (Elsevier, Netherlands) databases from inception to October 3, 2023, to identify longitudinal studies comparing the incidence of mortality in adult ambulatory patients with CCC and OC. No language restrictions were applied. We used the following search terms: Chagas cardiomyopathy, Chagas disease, Trypanosoma cruzi, American trypanosomiasis, mortality, and outcomes, among others. The complete search strategy is described in the supplementary data.
Study selection and eligibility criteriaWe included clinical trials and all observational studies (eg, cross-sectional, cohort, and case-control studies), except for case reports and case-series. We also excluded systematic reviews and meta-analyses. Studies that assessed adult patients with CCC and compared the mortality risk with those with OC were considered. The follow-up of included studies also had to be performed in an outpatient setting. We excluded studies in the pediatric population or animals.
Data screening and extractionThe selection process was carried out using Rayyan.6 Two independent reviewers screened the titles and abstracts, while considering the selection criteria. Any disagreements were resolved by a third reviewer. After this stage, full texts were reviewed to determine whether each study fulfilled the selection criteria. Relevant data from included studies was extracted using an Excel form. For studies reporting only medians and ranges (interquartile range, range, and maximum-minimum values), these values were converted into means and standard deviations using the method explained by Hozo, et al.7
Risk of bias assessmentThe study quality was independently evaluated by 2 authors employing the Newcastle-Ottawa Scale. In instances where a consensus was elusive, a third author arbitrated to reach a resolution. The quality of each study was analyzed on a 10-point scale, stratified into “low risk of bias” for 9 to 10 points, “medium risk of bias” for 6 to 8 points, and “high risk of bias” for scores less than 6.
Data synthesis and analysisThe summary measures for continuous variables are reported as the mean±standard deviation, while categorical variables are expressed as proportions. Participants were classified into 4 groups, initially: a) chronic Chagas cardiomyopathy (CCC); b) nonischemic cardiomyopathy (NICM), and c) ischemic cardiomyopathy (ICM). In addition, a fourth non-Chagas cardiomyopathy (non-CC) group was included for studies that did not characterize individuals in the comparator population (without Chagas cardiomyopathy) with respect to their etiology. Data synthesis was conducted by employing random effect models using the inverse variance method to estimate the pooled size effects, while the Paule-Mandel estimator was used to account for random error. In addition, the Hartung-Knapp adjustment was applied. Pooled unadjusted risk ratios (RR) were calculated from the mortality information reported, while adjusted hazard ratios (HR) were directly extracted from the multivariate models of the studies. Heterogeneity was assessed using the I2 statistic. Value higher than 75% were considered to indicate high heterogeneity, those between 25% and 75% as moderate, and those less than 25% as low. Meta-regression analysis was performed in contrasts with more than 10 studies to assess variables that might explain heterogeneity in the observed associations. A meta-regression analysis was also performed in contrasts with more than 10 studies to assess variables that might explain the heterogeneity in the observed associations, while a sensitivity analysis comparing studies published before and after 2016 was performed to explore the potential influence of the use of the more recently introduced angiotensin receptor-neprilysin inhibitors. Finally, publication bias was assessed using funnel plots and Egger's test. If potential publication bias was identified, the trim-and-fill method was employed using the metafor package to calculate an adjusted effect size, accounting for the presence of this bias. Statistical significance was set at a P<.05. All analyses were conducted using R Statistical Software (v4.2.3; R Core Team 2023) and the meta and metafor packages.
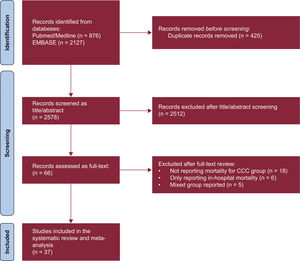
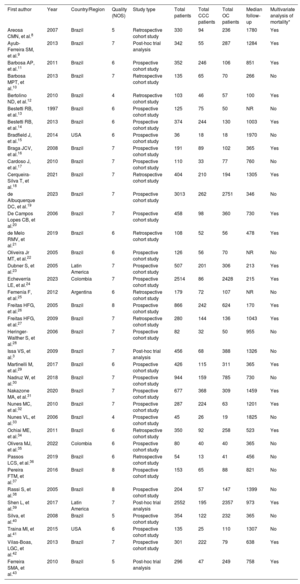
RESULTSStudy and patient characteristicsOut of 2134 screened studies, 37 met the selection criteria and were included in the meta-analysis (figure 1 and table 1).3,8–43 These studies were performed predominantly in Brazil (n=32, 86.5%) and were primarily prospective cohorts (64.9%). A total of 17 949 patients (4258 with CCC and 13 691 with OC) were analyzed. CCC patients were younger (mean difference, −2.35 years; 95% confidence interval [95%CI], −4.05 to −.65 years; I2, 93%) and less frequently male (odds ratio [OR], 0.74; 95%CI, 0.63-0.87; I2, 40%) compared with patients with OC, with no significant differences in the proportion of patients in New York Heart Association class III-IV status (OR, 0.88; 95%CI, 0.70-1.09; I2, 44.3%) or left ventricular ejection fraction (mean difference, 0.77%; 95%CI, −0.79% to 2.33%; I2, 90%). The mean follow-up times in the assessed studies ranged from 100 days to 1970 days (median, 758 days). Finally, most of the studies (86.5%) were classified as having a low risk of bias (figure 2A,B).
General characteristics of the included studies and the evaluated population
| First author | Year | Country/Region | Quality (NOS) | Study type | Total patients | Total CCC patients | Total OC patients | Median follow-up | Multivariate analysis of mortality* |
|---|---|---|---|---|---|---|---|---|---|
| Areosa CMN, et al.8 | 2007 | Brazil | 5 | Retrospective cohort study | 330 | 94 | 236 | 1780 | Yes |
| Ayub-Ferreira SM, et al.9 | 2013 | Brazil | 7 | Post-hoc trial analysis | 342 | 55 | 287 | 1284 | Yes |
| Barbosa AP, et al.11 | 2011 | Brazil | 6 | Prospective cohort study | 352 | 246 | 106 | 851 | Yes |
| Barbosa MPT, et al.10 | 2013 | Brazil | 7 | Retrospective cohort study | 135 | 65 | 70 | 266 | No |
| Bertolino ND, et al.12 | 2010 | Brazil | 4 | Retrospective cohort study | 103 | 46 | 57 | 100 | Yes |
| Bestetti RB, et al.13 | 1997 | Brazil | 6 | Prospective cohort study | 125 | 75 | 50 | NR | No |
| Bestetti RB, et al.14 | 2013 | Brazil | 6 | Prospective cohort study | 374 | 244 | 130 | 1003 | Yes |
| Bradfield J, et al.15 | 2014 | USA | 6 | Prospective cohort study | 36 | 18 | 18 | 1970 | No |
| Braga JCV, et al.16 | 2008 | Brazil | 7 | Prospective cohort study | 191 | 89 | 102 | 365 | Yes |
| Cardoso J, et al.17 | 2010 | Brazil | 7 | Prospective cohort study | 110 | 33 | 77 | 760 | No |
| Cerqueira-Silva T, et al.18 | 2021 | Brazil | 7 | Retrospective cohort study | 404 | 210 | 194 | 1305 | Yes |
| de Albuquerque DC, et al.19 | 2023 | Brazil | 7 | Prospective cohort study | 3013 | 262 | 2751 | 346 | No |
| De Campos Lopes CB, et al.20 | 2006 | Brazil | 7 | Prospective cohort study | 458 | 98 | 360 | 730 | Yes |
| de Melo RMV, et al.21 | 2019 | Brazil | 6 | Retrospective cohort study | 108 | 52 | 56 | 478 | Yes |
| Oliveira Jr MT, et al.22 | 2005 | Brazil | 6 | Prospective cohort study | 126 | 56 | 70 | NR | No |
| Dubner S, et al.23 | 2005 | Latin America | 7 | Prospective cohort study | 507 | 201 | 306 | 213 | Yes |
| Echeverría LE, et al.24 | 2023 | Colombia | 7 | Prospective cohort study | 2514 | 86 | 2428 | 215 | Yes |
| Femenía F, et al.25 | 2012 | Argentina | 6 | Retrospective cohort study | 179 | 72 | 107 | NR | No |
| Freitas HFG, et al.26 | 2005 | Brazil | 8 | Prospective cohort study | 866 | 242 | 624 | 170 | Yes |
| Freitas HFG, et al.27 | 2009 | Brazil | 7 | Retrospective cohort study | 280 | 144 | 136 | 1043 | Yes |
| Heringer-Walther S, et al.28 | 2006 | Brazil | 7 | Prospective cohort study | 82 | 32 | 50 | 955 | No |
| Issa VS, et al.3 | 2009 | Brazil | 7 | Post-hoc trial analysis | 456 | 68 | 388 | 1326 | No |
| Martinelli M, et al.29 | 2017 | Brazil | 6 | Prospective cohort study | 426 | 115 | 311 | 365 | Yes |
| Nadruz W, et al.30 | 2018 | Brazil | 7 | Prospective cohort study | 944 | 159 | 785 | 730 | No |
| Nakazone MA, et al.31 | 2020 | Brazil | 7 | Prospective cohort study | 677 | 368 | 309 | 1459 | Yes |
| Nunes MC, et al.32 | 2010 | Brazil | 7 | Prospective cohort study | 287 | 224 | 63 | 1201 | Yes |
| Nunes VL, et al.33 | 2006 | Brazil | 4 | Prospective cohort study | 45 | 26 | 19 | 1825 | No |
| Ochiai ME, et al.34 | 2011 | Brazil | 6 | Retrospective cohort study | 350 | 92 | 258 | 523 | Yes |
| Olivera MJ, et al.35 | 2022 | Colombia | 6 | Prospective cohort study | 80 | 40 | 40 | 365 | No |
| Passos LCS, et al.36 | 2019 | Brazil | 6 | Retrospective cohort study | 54 | 13 | 41 | 456 | No |
| Pereira FTM, et al.37 | 2016 | Brazil | 8 | Prospective cohort study | 153 | 65 | 88 | 821 | No |
| Rassi S, et al.38 | 2005 | Brazil | 8 | Prospective cohort study | 204 | 57 | 147 | 1399 | No |
| Shen L, et al.39 | 2017 | Latin America | 7 | Post-hoc trial analysis | 2552 | 195 | 2357 | 973 | Yes |
| Silva, et al.40 | 2008 | Brazil | 5 | Prospective cohort study | 354 | 122 | 232 | 365 | No |
| Traina MI, et al.41 | 2015 | USA | 6 | Prospective cohort study | 135 | 25 | 110 | 1307 | No |
| Vilas-Boas, LGC, et al.42 | 2013 | Brazil | 7 | Prospective cohort study | 301 | 222 | 79 | 638 | Yes |
| Ferreira SMA, et al.43 | 2010 | Brazil | 5 | Post-hoc trial analysis | 296 | 47 | 249 | 758 | Yes |
CCC, chronic Chagas cardiomyopathy; NOS, Newcastle-Ottawa Scale; NR, not reported; OC, other cardiomyopathies.
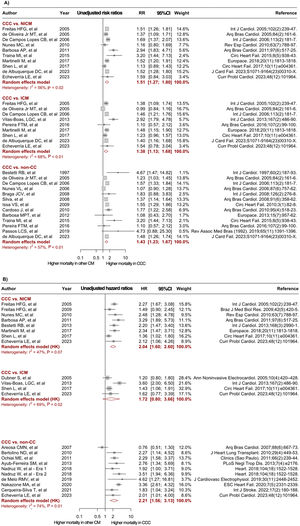
Forest plots comparing mortality risk between CCC and heart failure of other etiologies. A: unadjusted RR for CCC vs NICM, ICM, and non-CC. B: adjusted HR derived from multivariable Cox proportional-hazards models for CCC vs NICM, ICM, and non-CC. 95%CI, 95% confidence interval; CCC, chronic Chagas cardiomyopathy; CM; cardiomyopathy; HR, hazard ratio; ICM, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; NICM, nonischemic cardiomyopathy; non-CC, nonchagasic cardiomyopathy; NOS, Newcastle-Ottawa Scale; RR, risk ratio. The bibliographic references cited in the figure correspond to: Issa et al.,3 Areosa et al.,8 Ayub-Ferreira et al.,9 Barbosa et al.,11 Barbosa et al.,10 Bertolino et al.,12 Bestetti et al.,13 Bestetti et al.,14 Bradfield et al.,15 Braga et al.,16 Cardoso et al.,17 Cerqueira-Silva et al.,18 de Albuquerque et al.,19 De Campos Lopes et al.,20 de Melo et al.,21 Oliveira Jr et al.,22 Dubner et al.,23 Echeverría et al.,24 Femenía et al.,25 Freitas et al.,26 Freitas et al.,27 Heringer-Walther et al.,28 Martinelli et al.,29 Nadruz et al.,30 Nakazone et al.,31 Nunes et al.,32 Nunes et al.,33 Ochiai et al.,34 Olivera et al.,35 Passos et al.,36 Pereira et al.,37 Rassi et al.,38 Shen et al.,39Silva et al.,40Traina et al.,41Vilas-Boas et al.,42 Ferreira et al.43
In the unadjusted analyses, CCC patients had a significantly higher risk of mortality during follow-up compared with patients with NICM (RR, 1.44; 95%CI, 1.21-1.71; I2, 65%; 12 studies), ICM (RR, 1.34; 95%CI; 1.11-1.63; I2, 65%; 10 studies), and non-CC (RR, 1.42; 95%CI, 1.30-1.55; I2, 37%; 22 studies) (figure 2A). Twenty studies provided adjusted effect measures for the risk of mortality, mainly including age, sex, New York Heart Association class, left ventricular ejection fraction, and HF medications as adjustment covariates. The meta-analysis of adjusted measures reported a significantly higher mortality risk in the CCC group compared with the NICM (HR, 2.04; 95%CI, 1.60-2.60; I2, 47%; 8 studies) and the non-CC (HR 2.26; 95%CI, 1.65-3.10; I2, 71%; 11 studies) groups, while no significant effect was observed when patients with CCC and ICM were compared (HR, 1.72; 95%CI, 0.80-3.66; I2, 69%; 4 studies) (figure 2B). Of note, some studies reported information for more than one comparator group (ICM /NICM/non-CC), and consequently the total number of contrasts performed was higher than the number of articles included.
Meta-regression and publication biasThe results of meta-regression analysis revealed that none of the evaluated study characteristics (age, sex, New York Heart Association class, left ventricular ejection fraction, publication year, and follow-up time) was a significant source of heterogeneity (P>.05) (table 2 of the supplementary data). Moreover, a sensitivity analysis by publication year (before 2016 and after 2016) showed no significant differences in the effects between groups among the different contrasts (table 3 of the supplementary data). At the same time, Egger's test did not suggest publication bias in most of the performed analyses (P>.05), except for the unadjusted contrast between CCC and non-CC patients, which showed a potential bias toward reporting of larger effects (P=.024) (Figure 1 of the supplementary data). Nonetheless, after implementing the trim-and-fill method for this contrast, the adjusted estimates showed a consistently higher risk of mortality in the CCC group (RR, 1.39; 95%CI, 1.26-1.52; I2, 44%) (Figure 2 of the supplementary data).
DISCUSSIONIn the present meta-analysis, which evaluated more than 17 000 patients with HF, we observed that HF secondary to CCC was associated with a significantly higher mortality risk than HF arising from other causes. This result was evident in both unadjusted and adjusted models. Notably, we found a similar mortality risk in the meta-analysis of adjusted effects when comparing CCC and ICM. However, this finding was limited by a smaller number of studies (n=4) compared with the other adjusted contrasts (CCC vs NICM [n=8] and CCC vs non-CC [n=10]). Overall, our findings confirm the previously reported trend in individual studies and provide valuable information on the estimates for each etiologic group and the magnitude of the mortality risk associated with the diagnosis of CCC in HF patients. Interestingly, we observed a lower prevalence of male sex in the CCC group compared with the OC group. Current evidence suggests that male sex is associated with greater progression from the indeterminate form of Chagas disease to CCC44; however, there is also evidence suggesting that male sex is significantly associated with the development of OC, and the effect of this variable is potentially greater in other HF etiologies than in CCC.45–48
CCC represents a rapidly progressive form of cardiac involvement with a unique pathophysiology, characterized by severe immune cell infiltration to the myocardium, leading to extensive myocardial remodeling, intense fibrotic involvement, and a dilated cardiomyopathy phenotype.49 Moreover, transmural replacement of the myocardium by scar tissue is frequent in CCC and has been associated with the development of fatal arrhythmias, making sudden cardiac death the second cause of mortality in this population after worsening HF.50 In addition, systemic embolisms are highly incident in CCC due to the high prevalence of structural abnormalities, such as ventricular aneurysms and atrial fibrillation, as well as the presence of coagulation disorders intrinsic to chronic Trypanosoma cruzi infection.49,51 Finally, the neuroendocrine involvement observed during the course of the disease, marked by compromised adrenal and thymus gland function coupled with more pronounced parasympathetic denervation, may potentially explain the more severe clinical profile of this cardiomyopathy compared with other etiologies.52,53 Despite these insights, targeted therapeutic solutions that take into account the intricate pathophysiology of CCC remain elusive. Equally, there have been no dedicated randomized controlled trials evaluating the benefit of neurohormonal blockade in this patient population. Consequently, it is unknown whether patients with CCC benefit similarly from these therapies or continue to be at increased risk of mortality and adverse cardiovascular outcomes despite receiving optimal HF treatment.54,55 Therefore, the results of the PARACHUTE trial are eagerly anticipated, as they will compare sacubitril-valsartan with enalapril in patients with CCC, focusing on mortality risk and other cardiovascular outcomes. The results of this clinical trial may define the future of the pharmacological management of CCC and have the potential to set a new trend regarding mortality in this vulnerable population.56
LimitationsDespite its comprehensiveness, our study has several limitations. First, the moderate to high heterogeneity among the included studies in some comparisons, including various populations, methodologies, and clinical settings, could have introduced potential biases. Although difficult to determine, the observed interstudy heterogeneity may be due to multiple factors. These factors include the year of study publication. Although the year of publication was not significantly associated with the impact of CCC diagnosis on mortality in the meta-regression analyses, the relatively low sensitivity of this approach does not allow us to rule out the presence of a significant effect. This is because the diagnostic and therapeutic approaches to HF varied significantly between the publication of the first included study (1997) and the last study (2023). Other important factors include the type and stage of cardiac involvement, since some studies did not clarify the etiologies of the comparator groups or whether the individuals included had a diagnosis of HF or were in earlier stages of cardiomyopathy, as well as the mean follow-up time, which, although not significant in the meta-regression analysis, could also have influenced the differences observed. Finally, although most of the studies were performed in Brazilian populations, 7 studies (19%) were conducted in other countries, mainly Colombia and the United States. Differences in the distribution of Trypanosoma cruzi discrete typing units and other variables could have also influenced the observed results.
Second, we identified a potential publication bias in the studies reporting unadjusted risks for CCC vs non-CC groups. However, a significant effect was still observed after we adjusted by this type of bias using a trim-and-fill method. Moreover, the potential confounders included in the multivariate-adjusted models varied significantly among studies, as different covariate selection approaches were used. Nevertheless, the absence of significant results in the meta-regression analyses supports the accuracy of our results. Furthermore, the absence of patient-level data restricted our ability to conduct more refined subgroup analyses or assess the influence of individual patient characteristics on outcomes.
Of note, patients with CCC represent a vulnerable population, with limited access to health services and, therefore, to HF medications and therapies that reduce mortality, such as neurohormonal blockade and implantable cardioverter-defibrillators, which may influence their survival. However, we were unable to include data on socioeconomic status, access to HF therapies, or adherence to these drugs in our analyses, representing an important limitation.
Finally, assessment of all-cause mortality allowed the inclusion of a larger number of studies, not discriminating between the different causes of mortality (HF, sudden cardiac death, stroke, among others).
CONCLUSIONSThis meta-analysis indicates that CCC patients have an almost 2-fold increase in mortality risk during follow-up compared with their counterparts with HF secondary to OC. This finding underscores the pressing need to increase awareness of CCC prognosis and encourage the performance of large RCTs evaluating the benefit of HF therapies in this special population. Furthermore, our results invite further investigation of the factors potentially associated with the worse prognosis observed in patients with CCC, potentially highlighting access to HF therapies, treatment adherence, and early diagnosis of cardiomyopathy. Such insights are critical for shaping effective public policies and focusing research initiatives to better address the challenges of CCC and enhance outcomes for this vulnerable patient group.
- -
CCC is characterized by a unique pathophysiology that differentiates it from other etiologies of HF, potentially limiting the benefit of conventional diagnostic and therapeutic approaches to HF.
- -
CCC has been characterized by rapid progression and high mortality rate. Despite multiple studies highlighting worse clinical outcomes compared with other cardiomyopathies, there is lack of aggregated evidence analyzing whether diagnosis of CCC is associated with an increased risk of mortality.
- -
In this meta-analysis of 17 949 patients, those with CCC showed a consistently higher risk of mortality when compared with patients with other cardiomyopathies even after adjustment by relevant confounding covariates.
The present study did not require funding.
ETHICAL CONSIDERATIONSThe present work is exempt from approval by the institutional ethics committee because it corresponds to a systematic review of the literature and meta-analysis, which did not require patient recruitment or access to disaggregated information on individuals, since it was based on scientific publications freely available in the medical literature. Possible sex and gender biases have been taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence tools were used for the design or preparation of this study.
AUTHORS’ CONTRIBUTIONSS.A. Gómez-Ochoa participated in the study design, data collection, systematic review, study selection, methodology, statistical analysis, and manuscript review and editing. A.Y. Serrano-García oversaw the study design, data collection, systematic review, study selection, methodology, statistical analysis, and manuscript review and editing. A. Hurtado-Ortiz and A. Aceros were in charge of data collection, systematic review, study selection, and manuscript review. L.Z. Rojas and L.E. Echeverría oversaw the study design, study selection, and manuscript review and editing. All authors are responsible for reviewing the original manuscript and approving the final version.
CONFLICTS OF INTERESTThe authors declare that they have no conflicts of interest to disclose.