Up to 25% of patients who undergo a percutaneous coronary intervention show some limitation in the use of drug-eluting stents. The aim of this study was to evaluate if titanium-nitride-oxide–coated stents could be a good alternative to everolimus-eluting stents in diabetic patients.
MethodsA total of 173 diabetic patients with lesions at moderate risk of restenosis (exclusion criteria: diameter < 2.5 mm or length > 28 mm in vessels < 3mm, chronic occlusion) were randomized to a titanium group (83 patients) or an everolimus group (90 patients).
ResultsBaseline characteristics were well balanced; 28.3% of patients were insulin dependent. At 1 year, the incidence of major adverse cardiac events (death, nonfatal myocardial infarction, stroke, or repeat target vessel revascularization) was significantly higher in the titanium group than in the everolimus group (total, 14.5% vs 4.4%; P = .02; noninsulin-dependent subgroup, 9.7% vs 3.2%; P = .14; insulin-dependent subgroup, 28.6% vs 7.1%; P = .04). The incidence of death, nonfatal myocardial infarction, stroke, or any revascularization was 16.9% in the titanium group and 7.8% in the everolimus group (P = .06). Target lesion and vessel revascularizations occurred in 8.4% compared with 3.3% (P = .15) and in 13.3% compared with 3.3% (P = .01) in the titanium and everolimus groups, respectively. Angiographic follow-up at 9 months showed significantly less late lumen loss in the everolimus group (in-segment, 0.52 [standard deviation, 0.58) mm vs –0.05 [0.32] mm; in-stent, 0.76 [0.54] mm vs 0.13 [0.31] mm; P < .0001).
ConclusionsThe everolimus-eluting stent is superior to the titanium stent for clinical and angiographic end points in diabetic patients with lesions at moderate risk of restenosis.
Keywords
Implantation of drug-eluting stents (DES) is discouraged in approximately 25% of patients undergoing a percutaneous coronary intervention (PCI) due to situations that contraindicate prolonged dual antiplatelet therapy, such as chronic anticoagulation, history of bleeding, and planned interventions.1,2 This recommendation persists in guidelines, despite recent advances in DES that can limit the duration of dual antiplatelet therapy to 6 months.3
Although conventional stents are usually indicated in these patients, the situation is more complicated for patients with diabetes mellitus (DM). Therefore, DM is an accepted indication for DES use,4 due to the greater risk of restenosis found in studies that compared DES with older-generation conventional stents.5–9 Titanium-nitride-oxide (TiNOX)–coated stents have several potentially beneficial attributes, such as no release of chromium, nickel, and molybdenum, characteristics that have been linked with fewer fibrin deposits and a reduction in intimal hyperplasia, platelet adhesion, and inflammation.10–13 Some studies have reported lower rates of restenosis and occlusion for DES than conventional stents9,10 and fewer occlusions with paclitaxel-eluting stents in patients with acute myocardial infarction.12 TiNOX-coated stents and everolimus DES have also recently been compared in patients with acute coronary syndrome. At 12 months, noninferiority clinical trials showed no significant differences between them in target lesion revascularization (TLR): 6.5% vs 4.9%, respectively (P = .39).13 However, no randomized studies have compared TiNOX-coated stents and DES in diabetic patients predisposed to restenosis.
After positive results were obtained in a previous study by our group of TiNOX-coated stent use in diabetic patients,14 this randomized study was undertaken to compare a TiNOX-coated stent with a latest-generation DES—an everolimus-eluting stent (EES)— to determine if TiNOX-coated stents could be an equivalent alternative, at least for lesions not at high risk of restenosis. The TiNOX-coated stent results were also indirectly compared with those of conventional stents used in randomized studies with the same inclusion criteria as this study.
METHODSDesign and Patient SelectionThe multicenter, randomized, TITANIC XV trial compared patients with DM who underwent PCI with TiNOX-coated stents (Titan-2®, Hexacath, Paris, France) with those who received EES (Xience-V®, Abbott Vascular, Santa Clara, Illinois, United States). The inclusion criteria were as follows: diabetic patients, older than 18 years, and with at least 1 major de novo lesion (stenosis ≥ 50% of vessel diameter) in a native coronary artery. In each patient, all lesions were treated with the randomly assigned stent type. The exclusion criteria were as follows: pregnancy; allergy to acetylsalicylic acid, clopidogrel, heparin, or abciximab; active bleeding or risk of major bleeding; major renal failure (creatinine ≥ 2mg/dL); severe left ventricular dysfunction (ejection fraction ≤ 35%); cardiogenic shock; ST-elevation acute coronary syndrome in the first 48hours; ischemic stroke in the previous 6 months; contraindication for DES (eg, chronic anticoagulant treatment, planned surgery in the following 12 months); inability to provide informed consent; and life expectancy less than 12 months. Angiographic exclusion criteria were as follows: coronary artery disease, restenotic lesions, lesions that required a stent with a diameter < 2.5mm or > 3.5mm or a length > 28mm in vessels of less than 3mm, and chronic occlusions. Eligible patients were randomized to receive TiNOX-coated stents or EES in a 1:1 ratio. Group randomization was centralized and performed by an independent individual according to a table that was accessed for each referral to PCI. The study received no industry sponsorship. The study protocol was reviewed and approved by the ethics committees of all participating centers. All patients signed the corresponding informed consent. The study was conducted according to the ethical guidelines of the Declaration of Helsinki and is registered at ClinicalTrials.gov (NCT01510509).
Adjunctive Pharmacological TreatmentIf patients were already taking acetylsalicylic acid and/or clopidogrel, they received no additional loading dose. A dose of 300mg oral or 250 to 500 mg intravenous acetylsalicylic acid during the PCI and 100mg/day thereafter was given to the other patients. The clopidogrel loading dose was 600mg and 75mg/day thereafter. Clopidogrel was prescribed for at least 6 months to patients receiving EES and for at least 1 month to those receiving TiNOX-coated stents, which could be extended depending on cardiologist criteria. Unfractionated sodium heparin was administered during the procedure (100 mg/kg; 70 mg/kg if abciximab was coadministered). Abciximab use was left to investigator discretion, but in the protocol it was recommended for patients with acute coronary syndrome.
Clinical Follow-upPatients were prospectively followed up after discharge and at 1, 6, 12, and 24 months after the procedure. All data were collected in a shared electronic database that was reviewed at the end of follow-up for each patient. A clinical events committee recorded all clinical events in a blinded and independent manner.
Angiographic Follow-upA 9-month angiographic follow-up was performed only for those patients enrolled in the coordinating center. Two experienced and independent persons that were blinded to the assigned treatment analyzed the baseline, post-PCI, and 9-month follow-up angiographs with quantitative angiography (Xcelera®, Philips Healthcare, Best, The Netherlands). Quantitative angiography measurements of the target lesions were obtained in both the region of the stent and that of the segment (including the margins 5mm proximal and distal to the stent).
Study Definitions and VariablesStent implant in the target lesion was considered successful if there was < 20% residual stenosis and TIMI 3 flow, without dissection or thrombosis. The main clinical end point was major adverse cardiac events (MACE), defined as death, nonfatal acute myocardial infarction, stroke, or repeat target vessel revascularization (TVR) --MACE-1-- at 12 months of follow-up. Secondary end points included death, TLR, TVR, repeat revascularization of a vessel other than that of the target lesion, composite end point of death, nonfatal acute myocardial infarction, stroke, or repeat revascularization of any site (MACE-2); stent thrombosis; and clinical restenosis. Cardiac death was defined as death from cardiovascular or unknown causes. Myocardial infarction was diagnosed by the characteristic persistent chest pain with elevation of biochemical markers of myocardial necrosis (creatine kinase-MB fraction and troponin) at least twice the upper limit of the laboratory reference values and/or electrocardiographic criteria of appearance of pathological Q waves or ST segment deviations in at least 2 contiguous leads. Target lesion revascularization was defined as a new intervention (surgical or percutaneous) to treat luminal stenosis greater than 50% within the stent or in the segment 5mm proximal or distal to the stent after confirmation of ischemia. Target vessel revascularization was defined as revascularization due to ischemia secondary to disease of the target vessel. Overall revascularization included revascularization due to restenosis or progression due to arteriosclerosis. In the subgroup of patients with angiographic follow-up, late lumen loss (LLL) was defined as the difference between the minimum lumen diameter (MLD) after the stent implant procedure and the follow-up measurement. The main primary end point in subanalysis of this group was in-segment LLL at 9 months. Binary restenosis was defined as stenosis > 50% of the diameter of the target lesion. Stent thrombosis was defined according to the criteria of the Academic Research Consortium.
Statistical AnalysisThe sample size of this study had sufficient statistical power (beta risk, 20%), assuming superiority, to detect an absolute risk reduction of 15% in the principal event (assuming an 8% incidence of MACE in the EES group). Calculation of the sample size required for analysis of the primary end point in the angiograph subgroup (LLL at 9 months) was performed according to a noninferiority hypothesis, considering a difference > 0.4mm in the LLL to be clinically relevant. This noninferiority threshold was determined from previous studies demonstrating that LLL would have no clinical impact at less than 0.5 to 0.6mm,15 with an expected LLL of the EES group of approximately 0.15 mm. Given that the standard deviation of the LLL in previous studies is about 0.6 mm, at least 50 lesions in each treatment group (n=100) were required for an alpha risk of 2.5% (95% confidence interval [95%CI]) and a power of 85%.
Variables were analyzed according to the intention-to-treat principal, including all patients who underwent the index procedure. Continuous variables are presented as mean (standard deviation [SD]). Categorical variables are presented as absolute and relative frequencies. Between-group comparisons were performed using a Student t test for continuous variables and Pearson chi-squared or Fisher exact test for categorical variables. Survival curves, obtained with the Kaplan-Meier method, were compared with the log-rank test. Binary logistic regression and Cox regression were used to identify independent predictors of MACE. These models provided odds ratios (ORs) and rate ratios (RRs) with the corresponding 95%CI values. All independent variables found to be associated with the studied response (dependent) variable with P < .2 were included as covariates in the multivariate analysis. All tests were 2-tailed and were considered statistically significant at P < .05. All data were analyzed with SPSS version 16.
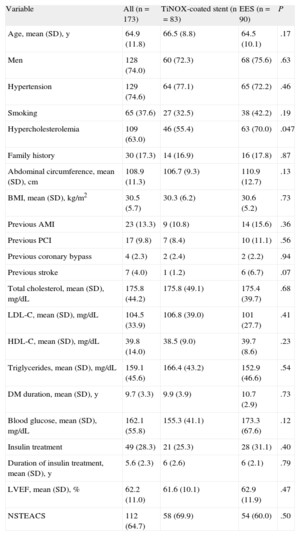
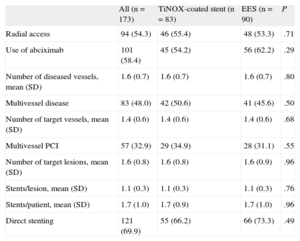
RESULTSBetween January 2009 and October 2011, a total of 173 patients were included from 8 centers (7 in Spain and 1 in Finland). The main baseline characteristics of the TiNOX-coated stent (83 patients, 124 lesions) and EES (90 patients, 134 lesions) groups are shown in Table 1. The mean age (standard deviation [SD]) was 64.9 (11.8) years; 74% were male and 28.3% were insulin dependent. The 2 groups were well balanced (except for the dyslipidemia variable), even in left ventricular ejection fraction and number of diseased vessels. A high percentage of patients (64.7%) had non–ST-elevation acute coronary syndrome. Procedure-related variables are shown in Table 2. More than half of the procedures were performed via a radial approach, and abciximab was used by 54.2% in the TiNOX-coated stent group and 62.22% in the EES group. There were no significant differences between the groups in the number of target lesions (1.6 [0.8]) and the number of stents per lesion (1.1 [0.3]) or per patient (1.7 [1.0]).
Baseline Characteristics of the Whole Group and by Randomization Group
| Variable | All (n=173) | TiNOX-coated stent (n=83) | EES (n=90) | P |
| Age, mean (SD), y | 64.9 (11.8) | 66.5 (8.8) | 64.5 (10.1) | .17 |
| Men | 128 (74.0) | 60 (72.3) | 68 (75.6) | .63 |
| Hypertension | 129 (74.6) | 64 (77.1) | 65 (72.2) | .46 |
| Smoking | 65 (37.6) | 27 (32.5) | 38 (42.2) | .19 |
| Hypercholesterolemia | 109 (63.0) | 46 (55.4) | 63 (70.0) | .047 |
| Family history | 30 (17.3) | 14 (16.9) | 16 (17.8) | .87 |
| Abdominal circumference, mean (SD), cm | 108.9 (11.3) | 106.7 (9.3) | 110.9 (12.7) | .13 |
| BMI, mean (SD), kg/m2 | 30.5 (5.7) | 30.3 (6.2) | 30.6 (5.2) | .73 |
| Previous AMI | 23 (13.3) | 9 (10.8) | 14 (15.6) | .36 |
| Previous PCI | 17 (9.8) | 7 (8.4) | 10 (11.1) | .56 |
| Previous coronary bypass | 4 (2.3) | 2 (2.4) | 2 (2.2) | .94 |
| Previous stroke | 7 (4.0) | 1 (1.2) | 6 (6.7) | .07 |
| Total cholesterol, mean (SD), mg/dL | 175.8 (44.2) | 175.8 (49.1) | 175.4 (39.7) | .68 |
| LDL-C, mean (SD), mg/dL | 104.5 (33.9) | 106.8 (39.0) | 101 (27.7) | .41 |
| HDL-C, mean (SD), mg/dL | 39.8 (14.0) | 38.5 (9.0) | 39.7 (8.6) | .23 |
| Triglycerides, mean (SD), mg/dL | 159.1 (45.6) | 166.4 (43.2) | 152.9 (46.6) | .54 |
| DM duration, mean (SD), y | 9.7 (3.3) | 9.9 (3.9) | 10.7 (2.9) | .73 |
| Blood glucose, mean (SD), mg/dL | 162.1 (55.8) | 155.3 (41.1) | 173.3 (67.6) | .12 |
| Insulin treatment | 49 (28.3) | 21 (25.3) | 28 (31.1) | .40 |
| Duration of insulin treatment, mean (SD), y | 5.6 (2.3) | 6 (2.6) | 6 (2.1) | .79 |
| LVEF, mean (SD), % | 62.2 (11.0) | 61.6 (10.1) | 62.9 (11.9) | .47 |
| NSTEACS | 112 (64.7) | 58 (69.9) | 54 (60.0) | .50 |
AMI, acute myocardial infarction; BMI, body mass index; EES, everolimus-eluting stent; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; NSTEACS, non–ST-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; SD, standard deviation; TiNOX, titanium-nitride-oxide.
Unless otherwise indicated, the data are expressed as No. (% of total).
Stent Implant Procedure Variables in Both Randomization Groups
| All (n=173) | TiNOX-coated stent (n=83) | EES (n=90) | P | |
| Radial access | 94 (54.3) | 46 (55.4) | 48 (53.3) | .71 |
| Use of abciximab | 101 (58.4) | 45 (54.2) | 56 (62.2) | .29 |
| Number of diseased vessels, mean (SD) | 1.6 (0.7) | 1.6 (0.7) | 1.6 (0.7) | .80 |
| Multivessel disease | 83 (48.0) | 42 (50.6) | 41 (45.6) | .50 |
| Number of target vessels, mean (SD) | 1.4 (0.6) | 1.4 (0.6) | 1.4 (0.6) | .68 |
| Multivessel PCI | 57 (32.9) | 29 (34.9) | 28 (31.1) | .55 |
| Number of target lesions, mean (SD) | 1.6 (0.8) | 1.6 (0.8) | 1.6 (0.9) | .96 |
| Stents/lesion, mean (SD) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) | .76 |
| Stents/patient, mean (SD) | 1.7 (1.0) | 1.7 (0.9) | 1.7 (1.0) | .96 |
| Direct stenting | 121 (69.9) | 55 (66.2) | 66 (73.3) | .49 |
EES, everolimus-eluting stent; PCI, percutaneous coronary intervention; SD, standard deviation; TiNOX, titanium-nitride-oxide.
Unless otherwise indicated, the data are expressed as No. (% of total).
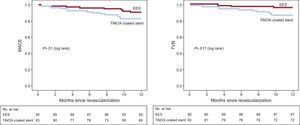
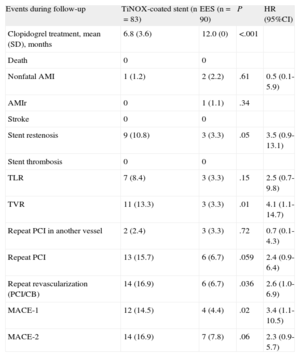
The 12-month clinical follow-up results are shown in Table 3. The incidence of MACE-1 (death, nonfatal acute myocardial infarction, stroke, or repeat TVR) was significantly higher in the TiNOX-coated stent group than in the EES group (14.5% vs 4.4%; P = .02; OR = 3.6; 95CI%, 1.1–11.7; HR = 3.4; 95%CI, 1.1-10.6). The incidence of MACE-2 (death, nonfatal acute myocardial infarction, stroke, or any revascularization) was 16.9% in the TiNOX-coated stent group and 7.8% in the EES group (TiNOX-coated stent: OR = 2.4; 95%CI, 0.9-6.3; HR = 2.3; 95%CI, 0.92-5.70; P = .06). Although TLR was more frequent in the TiNOX-coated stent group (8.4% vs 3.3%), the difference was not significant. However, there was a significant difference in the rates of TVR (13.3% vs 3.3%; P = .01) and repeat revascularization (16.9% vs 6.7%; P = .036). Survival curves of the different events are shown in the Figure.
Events After 1-Year Follow-up by Stent Type
| Events during follow-up | TiNOX-coated stent (n=83) | EES (n=90) | P | HR (95%CI) |
| Clopidogrel treatment, mean (SD), months | 6.8 (3.6) | 12.0 (0) | <.001 | |
| Death | 0 | 0 | ||
| Nonfatal AMI | 1 (1.2) | 2 (2.2) | .61 | 0.5 (0.1-5.9) |
| AMIr | 0 | 1 (1.1) | .34 | |
| Stroke | 0 | 0 | ||
| Stent restenosis | 9 (10.8) | 3 (3.3) | .05 | 3.5 (0.9-13.1) |
| Stent thrombosis | 0 | 0 | ||
| TLR | 7 (8.4) | 3 (3.3) | .15 | 2.5 (0.7-9.8) |
| TVR | 11 (13.3) | 3 (3.3) | .01 | 4.1 (1.1-14.7) |
| Repeat PCI in another vessel | 2 (2.4) | 3 (3.3) | .72 | 0.7 (0.1-4.3) |
| Repeat PCI | 13 (15.7) | 6 (6.7) | .059 | 2.4 (0.9-6.4) |
| Repeat revascularization (PCI/CB) | 14 (16.9) | 6 (6.7) | .036 | 2.6 (1.0-6.9) |
| MACE-1 | 12 (14.5) | 4 (4.4) | .02 | 3.4 (1.1-10.5) |
| MACE-2 | 14 (16.9) | 7 (7.8) | .06 | 2.3 (0.9-5.7) |
AMI, acute myocardial infarction; AMIr, AMI related with the target vessel; CB, coronary bypass; EES, everolimus-eluting stent; MACE, major adverse cardiac events; MACE-1, death, nonfatal AMI, stroke, or repeat revascularization of target vessel; MACE-2, death, nonfatal AMI, stroke, or any revascularization; PCI, percutaneous coronary intervention; SD, standard deviation; TiNOX, titanium-nitride-oxide; TLR, target lesion revascularization; TVR, target vessel revascularization.
Unless otherwise indicated, the data are expressed as No. (% of total).
Poorer results were seen in the subgroup of insulin-dependent patients, with greater differences between TiNOX-coated stents and EES in this group. The incidence of MACE-1 was higher in diabetic patients treated with TiNOX-coated stents than with EES (noninsulin-dependent diabetic patients, 9.7% vs 3.2%; P = .14; insulin-dependent diabetic patients, 28.6% vs 7.1%; P = .04). The incidence of MACE-2 was also higher in noninsulin-dependent diabetic patients (12.9% vs 9.7%; P = .57; insulin-dependent diabetic patients, 28.6% vs 7.1%; P =.045). In fact, insulin-dependent DM (OR = 2.9; P = .03), use of EES (OR = 0.25; P = .02), and age (OR = 6.09; P = .01) were independent predictors of a repeat PCI in multivariate analysis. The frequency of repeat PCIs was almost triple in insulin-dependent diabetic patients who received a TiNOX-coated stent (33.3% vs 10.3%; P = .04).
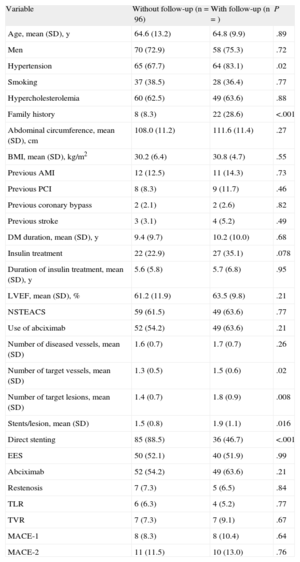
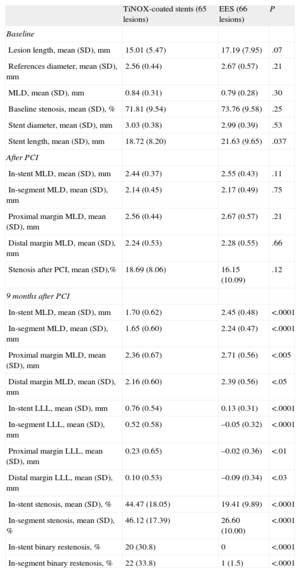
Angiographic Follow-upAngiographic follow-up was performed in 77 of the 80 patients (96.3%) included in the coordinating center (44.5% of the total group). There were no baseline differences between those patients who underwent angiographic follow-up and those who did not (Table 4). A total of 131 lesions were evaluated (65 and 66 in the TiNOX-coated stent and EES groups, respectively). The baseline data and postprocedural and 9-month measurements of the angiographic follow-up patients are summarized in Table 5. There were no significant differences between the groups in lesion length, reference diameter, MLD, baseline stenosis, and stent diameter and length. Similarly, there were no differences between the groups in the postprocedural measurements of the in-segment MLD or that of the stent margins. In-segment MLD at the 9-month follow-up was significantly higher in the EES group (1.65 [0.60] mm vs 2.24 [0.47] mm; P < .0001), and LLL was significantly lower in the EES group (in-stent LLL, 0.76 [0.54] mm vs 0.13 [0.31] mm; P < .0001; in-segment LLL, 0.52 [0.58] mm vs –0.05 [0.32] mm; P < .0001). The difference in the mean in-segment LLL, therefore, was 0.57 [0.08] (95%CI of the difference, 0.41-0.73) mm. Given that both limits of the 95%CI of the difference in the mean segment LLL values were greater than the noninferiority threshold established for this difference (0.4 mm), the noninferiority hypothesis is rejected.
Comparison of Baseline Characteristics and Events Between Patient Subgroups by Angiographic Follow-up
| Variable | Without follow-up (n=96) | With follow-up (n=) | P |
| Age, mean (SD), y | 64.6 (13.2) | 64.8 (9.9) | .89 |
| Men | 70 (72.9) | 58 (75.3) | .72 |
| Hypertension | 65 (67.7) | 64 (83.1) | .02 |
| Smoking | 37 (38.5) | 28 (36.4) | .77 |
| Hypercholesterolemia | 60 (62.5) | 49 (63.6) | .88 |
| Family history | 8 (8.3) | 22 (28.6) | <.001 |
| Abdominal circumference, mean (SD), cm | 108.0 (11.2) | 111.6 (11.4) | .27 |
| BMI, mean (SD), kg/m2 | 30.2 (6.4) | 30.8 (4.7) | .55 |
| Previous AMI | 12 (12.5) | 11 (14.3) | .73 |
| Previous PCI | 8 (8.3) | 9 (11.7) | .46 |
| Previous coronary bypass | 2 (2.1) | 2 (2.6) | .82 |
| Previous stroke | 3 (3.1) | 4 (5.2) | .49 |
| DM duration, mean (SD), y | 9.4 (9.7) | 10.2 (10.0) | .68 |
| Insulin treatment | 22 (22.9) | 27 (35.1) | .078 |
| Duration of insulin treatment, mean (SD), y | 5.6 (5.8) | 5.7 (6.8) | .95 |
| LVEF, mean (SD), % | 61.2 (11.9) | 63.5 (9.8) | .21 |
| NSTEACS | 59 (61.5) | 49 (63.6) | .77 |
| Use of abciximab | 52 (54.2) | 49 (63.6) | .21 |
| Number of diseased vessels, mean (SD) | 1.6 (0.7) | 1.7 (0.7) | .26 |
| Number of target vessels, mean (SD) | 1.3 (0.5) | 1.5 (0.6) | .02 |
| Number of target lesions, mean (SD) | 1.4 (0.7) | 1.8 (0.9) | .008 |
| Stents/lesion, mean (SD) | 1.5 (0.8) | 1.9 (1.1) | .016 |
| Direct stenting | 85 (88.5) | 36 (46.7) | <.001 |
| EES | 50 (52.1) | 40 (51.9) | .99 |
| Abciximab | 52 (54.2) | 49 (63.6) | .21 |
| Restenosis | 7 (7.3) | 5 (6.5) | .84 |
| TLR | 6 (6.3) | 4 (5.2) | .77 |
| TVR | 7 (7.3) | 7 (9.1) | .67 |
| MACE-1 | 8 (8.3) | 8 (10.4) | .64 |
| MACE-2 | 11 (11.5) | 10 (13.0) | .76 |
AMI, acute myocardial infarction; BMI, body mass index; DM, diabetes mellitus; EES, everolimus-eluting stent; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; MACE-1, death, nonfatal AMI, stroke, or repeat revascularization of target vessel; MACE-2, death, nonfatal AMI, stroke, or any revascularization; NSTEACS, non–ST-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; SD, standard deviation; TLR, target lesion revascularization; TVR, target vessel revascularization.
Unless otherwise indicated, the data are expressed as No. (% of total).
Stent and Lesion Data: Baseline, After Implant, and at 9 Months
| TiNOX-coated stents (65 lesions) | EES (66 lesions) | P | |
| Baseline | |||
| Lesion length, mean (SD), mm | 15.01 (5.47) | 17.19 (7.95) | .07 |
| References diameter, mean (SD), mm | 2.56 (0.44) | 2.67 (0.57) | .21 |
| MLD, mean (SD), mm | 0.84 (0.31) | 0.79 (0.28) | .30 |
| Baseline stenosis, mean (SD), % | 71.81 (9.54) | 73.76 (9.58) | .25 |
| Stent diameter, mean (SD), mm | 3.03 (0.38) | 2.99 (0.39) | .53 |
| Stent length, mean (SD), mm | 18.72 (8.20) | 21.63 (9.65) | .037 |
| After PCI | |||
| In-stent MLD, mean (SD), mm | 2.44 (0.37) | 2.55 (0.43) | .11 |
| In-segment MLD, mean (SD), mm | 2.14 (0.45) | 2.17 (0.49) | .75 |
| Proximal margin MLD, mean (SD), mm | 2.56 (0.44) | 2.67 (0.57) | .21 |
| Distal margin MLD, mean (SD), mm | 2.24 (0.53) | 2.28 (0.55) | .66 |
| Stenosis after PCI, mean (SD),% | 18.69 (8.06) | 16.15 (10.09) | .12 |
| 9 months after PCI | |||
| In-stent MLD, mean (SD), mm | 1.70 (0.62) | 2.45 (0.48) | <.0001 |
| In-segment MLD, mean (SD), mm | 1.65 (0.60) | 2.24 (0.47) | <.0001 |
| Proximal margin MLD, mean (SD), mm | 2.36 (0.67) | 2.71 (0.56) | <.005 |
| Distal margin MLD, mean (SD), mm | 2.16 (0.60) | 2.39 (0.56) | <.05 |
| In-stent LLL, mean (SD), mm | 0.76 (0.54) | 0.13 (0.31) | <.0001 |
| In-segment LLL, mean (SD), mm | 0.52 (0.58) | –0.05 (0.32) | <.0001 |
| Proximal margin LLL, mean (SD), mm | 0.23 (0.65) | –0.02 (0.36) | <.01 |
| Distal margin LLL, mean (SD), mm | 0.10 (0.53) | –0.09 (0.34) | <.03 |
| In-stent stenosis, mean (SD), % | 44.47 (18.05) | 19.41 (9.89) | <.0001 |
| In-segment stenosis, mean (SD), % | 46.12 (17.39) | 26.60 (10.00) | <.0001 |
| In-stent binary restenosis, % | 20 (30.8) | 0 | <.0001 |
| In-segment binary restenosis, % | 22 (33.8) | 1 (1.5) | <.0001 |
EES, everolimus-eluting stent; LLL, late lumen loss; MLD, minimum lumen diameter; PCI, percutaneous coronary intervention; SD, standard deviation; TiNOX, titanium-nitride-oxide.
Unless otherwise indicated, the data are expressed as No. (% of total).
Positive remodeling was seen at both proximal and distal borders of the EES group. Both in-stent and in-segment binary restenoses were significantly more frequent in the TiNOX-coated stent group than in the EES group (P > .0001). Finally, analysis of the possible effect of performing follow-up angiography, only in the coordinating center (44% of all patients and 50% of all lesions), revealed no significant differences in clinical restenosis or TLR between patients with and without follow-up angiography (6.55% vs 6.30%) (Table 4).
DISCUSSIONStudy ImportanceThe main finding of our study is that EES offer better results than TiNOX-coated stents in diabetic patients, even with the selection of lesions at moderate risk of restenosis. Thus, the principal end point of the study, MACE-1, reached statistical significance (14.5% vs 4.4%; P = .02). Incidence of the composite end point in the TiNOX-coated stent groups was almost double that of the EES group (16.9% vs 7.8%; P = .06), although this result was not statistically significant due to the small numbers of patients. There was no stent thrombosis in any group, but in the EES group there was a case of acute myocardial infarction in a target vessel.
Currently, more than 25% of patients who undergo a PCI have DM16; this percentage may increase in the medium term because the prevalence of DM is growing and is estimated to reach 10% of the adult population in the United States in the coming years.17 Recent meta-analyses have shown that the use of the new DES has reduced the percentage of restenosis in both the general and diabetic populations, without compromising safety, and the EES have shown better results than first-generation DES.18,19
Titanium stents are bioactive stents with specific properties that could provide certain advantages over conventional stents. Indeed, the titanium stent has received considerable attention recently because some studies have shown results superior to other conventional stents, while others have described it as equivalent to the DES in some patient populations. A randomized study by Moschovitis et al10 of de novo lesions in the general population showed a 9% need for revascularization at 5 years in the patient group that received TiNOX-coated stents, compared with 25% in the control group that received conventional stents. However, only 15% of the patients had diabetes.10 More recently, the BASE-ACS study of patients with acute coronary syndrome randomized 827 patients to receive either TiNOX-coated stents or EES. At 12 months, there were no significant differences in the incidence of MACE between the 2 groups (TiNOX-coated stents vs EES, 9.6% vs 9.0%; P = .89). The percentage of diabetic patients was also low (17%) in this study.13 Thus, a direct comparison between TiNOX-coated stents and latest-generation DES, such as EES, in a randomized study conducted specifically in diabetic patients is of great interest. To our knowledge, this work is the first randomized study to make such a direct comparison.
Our study showed that EES are superior to TiNOX-coated stents in diabetic patients, even when lesions at moderate risk of restenosis are selected. Angiography was only performed in the coordinating hospital, but there were no significant differences in the incidence of clinical restenosis or TLR (6.55% vs 6.30%) between patients receiving angiographic vs nonangiographic follow-up, which reflects the discipline of our protocol in only treating those patients with ischemia and also demonstrates the correlation between restenosis and ischemia, at least in this study.
Insulin-dependent patients have DM of a much longer duration and, thus, more severe coronary atherosclerosis.7 As expected, patients with insulin-dependent DM had a 2 to 3 times greater need for a repeat PCI and a greater probability of MACE, as seen in the multivariate analysis in the Results section identifying insulin-dependent DM as an independent predictor of a repeat PCI (OR = 2.9; P = .03), in agreement with other studies in this area.20 Repeat PCIs were 3 times more common in insulin-dependent DM patients who received a TiNOX-coated stent (33.3% vs 10.3%; P = .04), even though our study included a high percentage of patients treated with abciximab, particularly insulin-dependent diabetic patients (72.0% vs 52.8%). Insulin-dependent patients are those that benefit most from this treatment, according to previous results from our group.20 Thus, DES implantation in this subgroup of patients with insulin-dependent DM should be the treatment of choice whenever possible.
A positive remodeling effect (negative LLL in the proximal and distal borders) in the group of patients with EES could explain why there was more TVR in the TiNOX-coated stent group (13.3% vs 3.3%; P = .01). In-segment LLL with EES was –0.05mm due to positive remodeling at the proximal and distal margins. This interesting finding confirms that of previous studies such as the DIABETES trial,21 in which the borders of a sirolimus-eluting stent showed an intraluminal increase, with an increase in vessel volume, while the conventional stent group showed a negative remodeling effect and lumen reduction. Thus, in the sirolimus group, the antirestenotic effect extended to the edges. However, no positive remodeling effect was seen at the edges of the sirolimus-eluting stent in diabetic patients for paclitaxel DES.22 Our study shows the same type of positive effect with everolimus.
Indirect Comparisons of the Titanium Stent Results With Those of Other Studies of Similar DesignIndirect evidence suggests that TiNOX-coated stents could also function better than other conventional stents in diabetic patient populations. Between 2005 and 2008, 4 specific randomized trials were published that compared the Cypher sirolimus DES with the Bx Velocity conventional stent (both from Johnson & Johnson) in diabetic patients.5,6,8,9 These studies were very similar to each other and to the present study in the number of patients and the inclusion and exclusion criteria (Table 6). The in-segment LLL of the conventional stent in these 4 studies varied between 0.47mm and 1.02mm, with a mean of 0.76, with which the 0.52 in the TiNOX-coated stent group in our study compares favorably. Similarly, the mean incidence of clinical restenosis in the conventional stent group in these studies was 31.4%, which compares with 8.4% in our study.
Comparison With Other Randomized Studies Conducted in Diabetic Patients
| Studies/variables | DIABETES8 | SCORPIUS5 | DECODE9 | DESSERT6 | TITANIC XV |
| Convention stent groupa | 80 (BX-V), 110 lesions | 102 (BX-V) | 29 (BX-V), 47 lesions | 75 (BX-V), 109 lesions | 83 (Titan2), 65 lesions |
| DES groupa | 80 (SES), 111 lesions | 98 (SES) | 54 (SES), 81 lesions | 75 (SES), 109 lesions | 90 (Xience-V), 66 lesions |
| Insulin-dependent diabetic patients | 33 | 42 | 19.3 | 25.5 | 28.3 |
| Use of abciximab | 59 | NA | 30 | 100 | 63.1 |
| Inclusion criteria (D and L, mm) | D: 2.25-3.50; CTO: 13 | D: 2.5-3.5; L < 42 | D: 2.25-3.00; L < 23 | D: 2.5-3.5; L ≤ 28 | D: 2.5-3.5; L ≤ 28 if Ø < 3 |
| Vessel diameter, mean (SD), mm | 2.34 (0.6) | 2.60 (0.48) | 2.51 (0.35) | 2.66 (0.42) | 2.62 (0.50) |
| Lesion length, mean (SD), mm | 15.0 (8) | 11.35 (11.4) | 15.06 (6.34) | 14.9 (7.1) | 18.0 (5.53) |
| Stent diameter, mean (SD), mm | NA | NA | NA | 3 (0.4) | 3.04 (0.45) |
| Stent length, mean (SD), mm | 23 (12) | NA | 20.9 (8.45) | 19.9 (4.7) | 19 (7.2) |
| Lesions/patient, mean (SD) | 1.4 (0.6) | NA | 1.5 (0.67) | NA | 1.6 (0.9) |
| Stents/patient, mean (SD) | 1.6 (0.9) | 1.2 (0.47) | 2 (0.9) | NA | 1.71 (0.86) |
| Angiography | 9 months, in-segment LLL | 8 months, in-segment LLL | 6 months, in-stent LLL | 8 months, in-stent LLL | 9 months, in-segment LLL |
| Angiographic data | |||||
| In-segment LLL | |||||
| CS, mean (SD) | 0.47 (0.5) | 0.75 (0.59) | 1.09 (0.57) | 0.75 (0.66) | 0.52 (0.58) |
| DES, mean (SD) | 0.06 (0.4)b | 0.18 (0.45)b | 0.45 (0.65)b | 0.05 (0.36)b | –0.05 (0.32)b |
| In-segment binary restenosis | |||||
| CS | 33.7 | 42.1 | 57.1 | NA | 33.8 |
| DES | 7.8b | 8.8b | 12.8b | NA | 1.5b |
| In-stent binary restenosis | |||||
| CS | 31.7 | NA | 52.4 | 38.8 | 30.8 |
| DES | 3.9 | NA | 9b | 3.6b | 0b |
| Cardiac events | |||||
| AMI | |||||
| CS | 1.25 | 5 | 6.9 | 4.3 | 1.2 |
| DES | 0 | 4 | 1.9 | 1.5 | 2.2 |
| Death | |||||
| CS | 1.25 | 4 | 6.9 | 4.4 | 0 |
| DES | 1.25 | 5 | 0 | 2.9 | 0 |
| TLR | |||||
| CS | 31.3 | 30 | 34.5 | 30 | 8.4 |
| DES | 7.3b | 6b | 13b | 5.9b | 3.3 |
| TVR | |||||
| CS | NA | NA | 41.4 | 34.3 | 13.3 |
| DES | NA | NA | 14.8b | 14.7b | 3.3b |
| MACE | |||||
| CS | 36.3 | 35.8 | 41.4 | 40 | 8.4 |
| DES | 11.3b | 14.7 | 14.8b | 22.1 | 3.3 |
AMI, acute myocardial infarction; BX-V, Bx Velocity conventional stent; CS, conventional stent; CTO, chronic total occlusion; D, diameter; DES, drug-eluting stent; GPI, glycoprotein inhibitors IIb/IIIa; L, length; LLL, late lumen loss; MACE, major adverse cardiac events; NA, not available; PCI, percutaneous coronary intervention; SD, standard deviation; SES, sirolimus-eluting stent; TiNOX, titanium-nitride-oxide; TLR, target lesion revascularization.
Unless otherwise indicated, the data are expressed as %.
Studies in diabetic patients with the same inclusion criteria involving comparison of first-generation DES (and, accordingly, with greater LLL than current DES), such as the Endeavor® zotarolimus-eluting stent (Medtronic, Indianapolis, Indiana, United States) and the Taxus® paclitaxel-eluting stent (Boston Scientific, Indianapolis, Indiana, United States),23 found percentages of restenosis of 6.9% and 5.8%, respectively, a reasonably favorable comparison with our results of 8.4% restenosis with TiNOX-coated stents. Although these data are interesting, they are derived from indirect comparisons and should thus be interpreted with caution.
Study LimitationsAlthough the number of patients in the current study was relatively low, particularly for clinical events such as stent thrombosis, the main study objectives were clearly addressed. The lesions were at moderate risk of restenosis, and the differences would certainly have been higher upon inclusion of lesions at higher risk of restenosis. The TiNOX-coated stent results compared favorably with other conventional stents of other similarly designed studies in patients with DM. Although caution must be used when interpreting indirect comparisons, the LLL of TiNOX-coated stents measured in the present study is very similar to that of another study of TiNOX-coated stents in DM patients. The subanalysis results of the insulin-dependent group should be seen as illustrative because the results are limited by the post-hoc analysis.
Another limitation is the possible difference in the duration of dual antiplatelet therapy, but this difference would not affect the TLR and would certainly be reduced in patients with acute coronary syndrome, who are usually maintained on this therapy by general cardiologists for 6 months. Moreover, there were no differences in other lesions apart from those of the target vessel.
CONCLUSIONSIn patients with diabetes, even with lesions at moderate risk of restenosis, EES were found to be superior to TiNOX-coated stents, with lower incidences of LLL, TVR, and MACE. This difference was particularly marked in patients with insulin-dependent DM. The favorable results of TiNOX-coated stents compared with other conventional stents or with EES for acute coronary syndrome cannot be extrapolated to the diabetic population, in which the use of latest-generation DES whenever possible is always recommended.
FUNDINGThis work was supported by a grant from the Extremadura Society of Cardiology Association (Asociación Sociedad Extremeña de Cardiología).
CONFLICTS OF INTERESTNone declared.
| Hospital Universitario Infanta Cristina, Badajoz, Spain | Reyes González-Fernandez, Ginés Martínez-Cáceres, José R. López-Mínguez, Juan M. Nogales-Asensio, Luis J. Doncel-Vecino, Antonio Merchán-Herrera |
| Hospital Universitario General, Valencia, Spain | Francisco Pomar-Domingo |
| Hospital Puerto Real, Cádiz, Spain | Pedro Martínez-Romero |
| Hospital Puerta de Hierro, Madrid, Spain | José A. Fernández-Díaz |
| Hospital Virgen de la Arrixaca, Murcia, Spain | Raúl Valdesuso-Aguilar |
| Hospital Virgen de la Salud, Toledo, Spain | José Moreu-Burgos |
| Hospital Juan Ramón Jiménez, Huelva, Spain | José Díaz-Fernández |
| Heart Center, Satakunta, Pori, Finland | Pasi Karjalainen |