The Kansas City Cardiomyopathy Questionnaire (KCCQ) is specifically designed to evaluate quality of life in patients with chronic heart failure (CHF). The purpose of this study was to assess the reliability, validity, and responsiveness to change of the Spanish version of the KCCQ.
MethodsThe multicenter study involved 315 patients with CHF. Patients were evaluated at baseline and at weeks 24 and 26. The KCCQ, the Minnesota Living with Heart Failure Questionnaire (MLHFQ), and the Short Form-36 (SF-36) were administered. Reliability was assessed in stable patients (n=163) by examining test–retest and internal consistency measures between weeks 24 and 26. Validity was evaluated at baseline (n=315) by determining how KCCQ scores varied with New York Heart Association (NYHA) functional class and by comparing scores with those on similar domains of the MLHFQ and SF-36. Responsiveness to change was assessed in patients who experienced significant clinical improvement between baseline and week 24 (n=31) by determining the effect size.
ResultsReliability coefficients ranged between 0.70 and 0.96 for the different domains. Mean KCCQ scores varied significantly with NYHA functional class (P<.001). Correlations with comparable domains on the other questionnaires were acceptable (e.g. for physical limitation, they were between 0.77 and 0.81). The changes observed at 24 weeks in the majority of KCCQ scores in the subsample that improved corresponded to a moderate effect size (i.e. 0.4–0.6).
ConclusionsThe Spanish version of the KCCQ has good metric properties (i.e. validity, reliability and responsiveness), which make it suitable for use in evaluating quality of life in Spanish CHF patients.
Keywords
Chronic heart failure (CHF) has become a major burden for health systems because of its high prevalence, high mortality, and high associated costs, particularly those related to the elevated hospitalization rate.1,2 In addition, the functional capacity of patients with CHF is seriously limited, which in turn limits their activities of daily living.3,4
Treatment of CHF is associated with decreased mortality and fewer hospital admissions, as well as an improvement in patients’ symptoms and well-being.2 There is, therefore, growing interest in assessing patient-reported health outcomes.5,6 Health professionals usually monitor patients using objective measures of ventricular function (e.g. echocardiography or natriuretic peptide levels)2 or of functional capacity (e.g. New York Heart Association [NYHA]7 functional class, the 6-min walk test,8 or exercise testing with gas exchange analysis).2 However, these traditional indicators are only weakly correlated with the patients’ own perception of their disease, while, from the physician's perspective, their availability is limited, they are expensive, and, in the case of the NYHA functional class, they may offer only a subjective assessment.9,10 In contrast, health-related quality-of-life (HRQL) measures provide direct information about the patients’ own perception of how CHF affects their well-being and daily activities.3,10 These HRQL measures provide additional information that cannot be obtained by traditional clinical or functional measures of CHF.
A number of different HRQL questionnaires have been specifically developed for patients with CHF.11,12,13,14,15,16,17 Of these, the Kansas City Cardiomyopathy Questionnaire (KCCQ)11 is the most recent and the only one that, in addition to assessing domains classically covered by such questionnaires (i.e. physical, symptom and social domains), also incorporates an assessment of the change in symptoms and in the level of the patients’ self-efficacy and knowledge. Although the KCCQ has been shown to have acceptable capacity for measuring HRQL in different studies,2,18 the linguistic adaptation of the Spanish version has not yet been assessed.
The objective of the present study was to assess the feasibility, reliability, validity, and sensitivity to change of the Spanish version of the KCCQ when used in regular clinical practice in outpatient clinics specialized in CHF.
Methods Study DesignThe VALIC-KC (Spanish abbreviation for Validation of the Spanish Version of the Kansas City Cardiomyopathy Questionnaire Quality-of-Life Questionnaire in Patients with Heart Failure) was a prospective cohort study in patients with CHF. Consecutive patients were recruited from outpatient clinics specialized in CHF (i.e. in cardiology and internal medicine departments) at 34 Spanish hospitals.
Consecutive patients who met European Cardiology Society2 diagnostic criteria for mild-to-moderate CHF were considered for inclusion. All patients had to give informed consent in writing and to meet the following inclusion criteria: (a) left ventricular ejection fraction <35% or admission to hospital for documented CHF in the past year; (b) stable clinical condition; (c) therapeutic optimization of CHF foreseen according to European guidelines2; and (d) ability to follow a protocol. The following exclusion criteria were applied: (a) admission to hospital in the past 4 weeks; (b) acute decompensation requiring admission to hospital; (c) noncardiac disease with a life expectancy of less than 1 year; (d) psychiatric illness interfering with an appropriate follow-up; (e) CHF due to severe primary (uncorrected) valve disease; (f) significant liver or kidney disease; (g) history of stroke in the past 3 months; and (h) motor limitation preventing the performance of the 6-min walk test. The study was approved by the relevant ethics committees.
After the baseline visit, patients attended follow-up visits at weeks 24 and 26 after inclusion. At the baseline visit, patients performed a 6-min walk test, and underwent evaluations of cognitive function19 and social support,20 and their HRQL was assessed using the KCCQ, the Short Form-36 (SF-36), and the Minnesota Living with Heart Failure Questionnaire (MLHFQ). At visit 2 (24 weeks), all clinical events occurring since inclusion were assessed and the tests and questionnaires used at baseline were administered again. Two weeks after visit 2, patients attended the final study visit (visit 3, 26 weeks), whose function was to determine whether the patient had remained clinically stable with respect to visit 2, thereby enabling the reproducibility of the HRQL questionnaires to be assessed. During this final visit, the same information was collected as in visit 2, although the 6-min walk test was not performed.
Quality-of-Life QuestionnairesThe KCCQ11 is a self-administered HRQL questionnaire specific to patients with CHF. It comprises 23 items in seven domains: physical limitation; symptoms, with domains for change over time, frequency, and severity; self-efficacy and knowledge; quality of life; and social interference. The response options for the items are Likert-type scales ranging from 1 to 5, 6 or 7 points and the score on each domain can, in theory, range from 0 to 100, with 100 corresponding to the best state. In addition, two summary scores are calculated: the clinical summary score is derived by summing the individual scores on the physical limitation and symptoms domains (i.e. total symptom score) with the change of symptoms over time excluded; and the overall summary score is derived by summing the clinical summary score and the quality of life and social interference scores.
The MLHFQ13 is a self-administered questionnaire that contains 21 items, provides an overall score and has two domains: physical and emotional. The response options range from 0, indicating no effect on HRQL, to 5, indicating the maximum effect. The questionnaire scores, both overall (i.e. 0–105) and by domain (i.e. physical 0–40 and emotional 0–25), are obtained by summing responses to the individual items.
The SF-36 questionnaire is a general health questionnaire21,22,23 which comprises 36 questions that measure eight health scales (i.e. physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health24) and includes two summary measures: mental and physical.25 A higher score indicates a better state of health.
Following the recommendations of the authors of the original versions of these questionnaires, scores for missing values were obtained by imputation, provided that less than half the items were missing on a given score.
Subsamples for Estimating Validity, Reliability, and Sensitivity to ChangeIn order to assess validity and internal consistency, a total patient sample comprising all those included in the first study visit was analyzed (i.e. 315 patients). Reproducibility between visits 2 and 3 was assessed in stable patients (i.e. 163 patients). To assess sensitivity to change, patients were classified according to the clinical changes occurring between visits 1 and 2. Changes considered clinically relevant were a change of at least one category in NYHA functional class and a difference greater than 10% in the distance covered in the 6-min walk test, as defined in previous studies.26,27,28,29,30 A stability subsample included patients who did not meet either of the aforementioned criteria for a relevant clinical change between visits 1 (week 0) and 2 (week 24).
Statistical AnalysisThe differences between subsamples and the original sample were analyzed using either parametric or nonparametric tests for continuous variables according to their distribution or using the χ2 test for categorical variables.
For each questionnaire, the following were evaluated: the range of scores observed, the percentage of patients with an unanswered item in each domain (as a measure of feasibility) and the percentage of patients with either a maximum score (as a measure of the ceiling effect) or a minimum score (as a measure of the floor effect). Reliability was assessed by calculating the internal consistency, which was estimated using the Cronbach α coefficient,31 and by analyzing test–retest reproducibility using the intraclass correlation coefficient.32 The Cronbach α coefficient gives a measure of the homogeneity between items in a domain in a single test administration and was determined using the initial evaluation of the entire sample. The intraclass correlation coefficient is a measure of concordance, and was calculated using data obtained from patients who were stable between the last two visits (i.e. weeks 24 and 26). Both the Cronbach α and the intraclass correlation coefficient took values between 0 and 1. A value of 0.7 was the standard proposed for intergroup comparisons, whereas for comparisons between individuals, a coefficient of 0.9 was considered appropriate.33
Construct validity was evaluated by analyzing the relationships between the scores for similar domains of the HRQL questionnaires and between questionnaire scores and related clinical measures33: (1) a correlation matrix (i.e. a multitrait multimethod matrix) was used to compare domains of the KCCQ, MLHFQ, and SF-3634 with a priori convergent and discriminatory hypotheses of validity being defined between similar and different domains, respectively; (2) the relationship between the pattern of scores on the KCCQ and NYHA functional class was assessed using the Cuzick nonparametric linear trend test; and (3) the association between the domain of physical limitation and either functional class or the distance covered in the 6-min walk test was analyzed using the Spearman correlation coefficient.
The sensitivity of the KCCQ to change was analyzed by comparing the means of the scores between visits 1 and 2 (Wilcoxon test) and an effect size coefficient was constructed (i.e. mean of the change/initial standard deviation) from changes in the scores.35 An effect size greater than 0.8 was considered as large, one of 0.5 as moderate, and one close to 0.2 as small.
The analysis was performed using SPSS 17.0 software for Windows (Chicago, IL, USA).
ResultsIn total, 315 patients were included at visit 1. These patients formed the initial sample for determining the validity of the questionnaire (visit 1, week 0). Subsequently, 300 patients attended visit 2 (week 24): there were 8 no-shows and 7 had died. Of these 300, 31 had improved clinically and formed the subsample for analyzing sensitivity to change. At visit 3 (week 26), 296 patients attended: 4 were no-shows. Of these 296, 163 had remained stable between visits 2 and 3 and formed the subsample for the analysis of reliability.
Table 1 shows the sociodemographic and baseline clinical characteristics of all patients included in the study and of patients in the subsamples used for analyzing sensitivity to change. Compared with the overall sample, the improvement subsample contained a smaller proportion of widowed patients (P=.01), had a poorer mean functional class (P<.001), and covered a shorter mean distance on the 6-min walk test (P<.05). For the stability subsample, there were differences in functional class (P=.001) and in the proportion of patients with dyslipidemia (P=.013).
Table 1. Sociodemographic and Baseline Clinical Characteristics of All Patients and Two Subsamples.
| All patients (n=315) | Subsample for sensitivity to change analysis | ||
| Improvement (n=31) | Stability (n=72) | ||
| Sex | |||
| Male | 233 (74.0%) | 24 (73.3%) | 58 (80.6%) |
| Female | 82 (26.0%) | 7 (23.3%) | 14 (19.4%) |
| Age, years | 64.5 (12.2) | 62.4 (12.9) | 63.1 (12.6) |
| m.v. age | 8 (2.5%) | 2 (6.5%) | 1 (1.4%) |
| Marital status | |||
| Partner | 216 (69.9%) | 22 (73.3%) | 52 (74.3%) |
| Single/divorced/separated | 34 (11.0%) | 7 (23.3%) | 8 (11.4%) |
| Widow(er) | 59 (19.1%) | 1 (3.3%) | 10 (14.3%) |
| m.v. marital status | 6 (1.9%) | 1 (3.2%) | 2 (2.8%) |
| Cause of heart failure | |||
| Ischemic | 113 (36.5%) | 9 (29.0% | 29 (40.8%) |
| Nonischemic | 197 (63.5%) | 22 (71.0%) | 42 (59.2%) |
| m.v. cause | 5 (1.6%) | 0 (0.0%) | 1 (1.4%) |
| NYHA class | |||
| I | 24 (8.3%) | 0 (0.0%) | 5 (6.9%) |
| II | 188 (64.8%) | 16 (51.6%) | 60 (83.3%) |
| III | 75 (25.9%) | 12 (38.7%) | 7 (9.7%) |
| IV | 3 (1.0%) | 3 (9.7%) | 0 (0.0%) |
| m.v. NYHA class | 25 (7.9%) | 0 (0.0%) | 0 (0.0%) |
| Comorbidities | |||
| HT | 186 (59.6%) | 21 (67.7%) | 40 (55.6%) |
| m.v. HT | 3 (1.0%) | 0 (0.0%) | 0 (0.0%) |
| Dyslipidemia | 145 (46.9%) | 10 (32.3%) | 43 (59.7%) |
| m.v. dyslipidemia | 6 (1.9%) | 0 (0.0%) | 0 (0.0%) |
| Chronic renal insufficiency a | 31 (10.5%) | 0 (0.0%) | 8 (12.1%) |
| COPD | 48 (15.6%) | 7 (22.6%) | 8 (11.1%) |
| Cognitive functioning | |||
| Adjusted Pfeiffer score | 1.2 (1.7) | 1.3 (1.9) | 1.3 (1.7) |
| Cognitive decline (adjusted) | 70 (22.7%) | 5 (17.9%) | 8 (11.3%) |
| 6-MWT distance, m | 367.9 (173.2) | 309.5 (141.3) | 382.4 (135.5) |
| LVEF, % | 32.3 (13.1) | 32.3 (12.9) | 32.4 (12.9) |
| Treatment | |||
| ACE inhibitors | 238 (77.3%) | 24 (77.4%) | 57 (79.2%) |
| ARA-II | 70 (24.3%) | 7 (28.0%) | 15 (22.1%) |
| β-Blockers | 262 (85.6%) | 22 (75.9%) | 65 (90.3%) |
| Diuretics | 267 (86.1%) | 27 (90.0%) | 58 (81.7%) |
Categoric variables are expressed as n (%) and continuous variables, as mean (standard deviation).
Abbreviations: 6MWT, 6-min walk test; ACE, angiotensin-converting enzyme; ARA-II, angiotensin receptor-II agonist; COPD, chronic obstructive pulmonary disease; HT, hypertension; LVEF, left ventricular ejection fraction; m.v., missing values; NYHA, New York Heart Association.
a Renal insufficiency was defined as a serum creatinine level >1.5mg/dL.
The proportion of patients with a missing item on the KCCQ was high (i.e. 27.9%; Table 2). Nevertheless, by treating missing values as proposed by the questionnaire's original authors, imputed scores were obtained for most patients. In contrast, approximately 3% of patients had missing values for all scores on the MLHFQ.
Table 2. Distribution of Scores and Reliability Coefficients of the Kansas City Cardiomyopathy Questionnaire, the Minnesota Living With Heart Failure Questionnaire, and the Short Form-36, Calculated With the Initial Sample (n=315).
| Scales | Mean (SD) | Items with m.v. (%) | Dimensions with m.v. (%) | Observed range | Floor (%) | Ceiling (%) | Cronbach α | ICC a |
| KCCQ | ||||||||
| Physical limitation | 70.1 (25.7) | 10.5 | 0.6 | 0–100 | 0.3 | 10.9 | 0.90 | 0.92 (0.89–0.94) |
| Symptom stability | 56.0 (23.0) | 0.0 | 0.0 | 0–100 | 4.1 | 12.4 | – | 0.68 (0.59–0.76) |
| Symptom frequency | 75.0 (24.6) | 4.1 | 0.6 | 0–100 | 0.3 | 23.6 | 0.80 | 0.93 (0.91–0.95) |
| Symptom burden | 76.8 (23.1) | 1.0 | 0.0 | 0–100 | 0.3 | 28.3 | 0.80 | 0.88 (0.85–0.91) |
| Overall symptoms | 76.0 (23.2) | 4.4 | 0.0 | 3.1–100 | 0.0 | 21.3 | 0.88 | 0.92 (0.90–0.94) |
| Self-efficacy | 73.4 (24.3) | 2.2 | 1.0 | 0–100 | 1.0 | 25.0 | 0.70 | 0.87 (0.83–0.91) |
| Quality of life | 59.0 (25.7) | 1.0 | 0.6 | 0–100 | 2.2 | 7.0 | 0.83 | 0.90 (0.86–0.92) |
| Social interference | 66.3 (28.8) | 19.0 | 2.2 | 0–100 | 1.9 | 19.5 | 0.89 | 0.89 (0.85–0.92) |
| Overall summary | 68.0 (23.0) | 27.9 | 0.0 | 1.8–100 | 0.0 | 2.9 | 0.96 | 0.94 (0.92–0.96) |
| Clinical summary | 73.1 (23.0) | 14.6 | 0.0 | 3.7–100 | 0.0 | 8.6 | 0.94 (0.92–0.96) | |
| MLHFQ | ||||||||
| Physical domain | 14.6 (10.7) | 3.5 | 3.2 | 0–39 | 0.0 | 8.9 | 0.93 | |
| Emotional domain | 8.5 (6.4) | 1.0 | 3.2 | 0–25 | 0.7 | 9.8 | 0.87 | |
| Social domain | 7.1 (5.5) | 6.7 | 3.2 | 0–20 | 0.3 | 17.7 | 0.75 | |
| Total | 36.0 (23.6) | 10.5 | 3.2 | 0–95 | 0.0 | 1.3 | 0.94 | |
| SF-36 | ||||||||
| Physical functioning | 54.8 (26.8) | 4.1 | 1.0 | 0–100 | 1.6 | 2.6 | 0.92 | |
| Role-physical | 56.6 (30.9) | 2.5 | 1.6 | 0–100 | 5.2 | 15.8 | 0.93 | |
| Bodily pain | 70.0 (25.1) | 2.5 | 1.6 | 12–100 | 0.0 | 30.0 | 0.85 | |
| General Health | 43.0 (20.3) | 4.1 | 2.2 | 0–97 | 1.0 | 0.0 | 0.74 | |
| Vitality | 51.5 (23.9) | 2.9 | 1.9 | 0–100 | 2.3 | 4.2 | 0.82 | |
| Social functioning | 69.7 (27.9) | 3.8 | 0.6 | 0–100 | 1.9 | 30.4 | 0.82 | |
| Role-emotional | 75.0 (27.0) | 3.8 | 2.5 | 0–100 | 3.3 | 38.1 | 0.93 | |
| Mental health | 64.5 (21.3) | 4.1 | 1.9 | 0–100 | 1.0 | 5.5 | 0.84 | |
| Physical summary | 40.2 (9.0) | – | 3.8 | 17.7–61.0 | – | – | – | |
| Mental summary | 46.3 (12.0) | – | 3.8 | 4.7–70.6 | – | – | – | |
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living with Heart Failure Questionnaire; m.v., missing values; SD, standard deviation; SF-36, Short Form-36.
a The intraclass correlation coefficient (ICC) was calculated in patients who were stable between visits 2 and 3 (n=163).
Observed scores on the KCCQ and SF-36 were distributed broadly over the entire range available. For the MLHFQ, however, the observed range of scores was identical to the theoretical range in only the emotional domain (Table 2). The floor effect percentages were generally low for the KCCQ, MLHFQ and SF-36, whereas the roof effect percentages were high for the symptom burden item of the KCCQ and for the domains of bodily pain, social functioning and role-emotional of the SF-36.
Validity AnalysisThe correlation matrix for the KCCQ domains and the domains of the other two questionnaires shows that most domains classified as convergent by our initial hypothesis (marked in boldface in Table 3) had values greater than 0.5. For example, the correlations between the KCCQ symptoms domains and the physical domain of the MLHFQ were all close to 0.8, and the KCCQ social interference domain showed a correlation of 0.7 with the SF-36 social functioning domain. The correlations between the KCCQ domains of symptom stability and self-efficacy and knowledge and the other domain scores, which were defined a priori as being discriminatory (in italics in Table 3), were low, ranging from 0.005 to 0.193 and 0.183 to 0.440, respectively.
Table 3. Spearman Correlation Matrix (Multitrait Multimethod Matrix) for Assessing the Validity of the Kansas City Cardiomyopathy Questionnaire (n=315).
| KCCQ domains and summary scores | ||||||||||
| PL | SS | SF | SB | OSY | SE | QoL | SL | OSU | CS | |
| MLHFQ | ||||||||||
| Physical domain | −0.772 | −0.193 | −0.781 | −0.788 | −0.808 | −0.440 | −0.711 | −0.735 | −0.845 | −0.839 |
| Emotional domain | −0.589 | −0.109 | −0.583 | −0.614 | −0.614 | −0.374 | −0.681 | −0.616 | −0.706 | −0.637 |
| Social domain | −0.499 | −0.094 | −0.521 | −0.533 | −0.542 | −0.292 | −0.589 | −0.613 | −0.634 | −0.548 |
| Total | −0.721 | −0.134 | −0.725 | −0.737 | −0.753 | −0.414 | −0.749 | −0.747 | −0.836 | −0.781 |
| SF-36 | ||||||||||
| Physical functioning | 0.811 | 0.178 | 0.695 | 0.679 | 0.706 | 0.400 | 0.620 | 0.676 | 0.778 | 0.812 |
| Role-physical | 0.593 | 0.133 | 0.587 | 0.601 | 0.609 | 0.350 | 0.605 | 0.673 | 0.701 | 0.629 |
| Bodily pain | 0.436 | −0.005 | 0.374 | 0.371 | 0.388 | 0.183 | 0.356 | 0.400 | 0.447 | 0.437 |
| General health | 0.409 | 0.187 | 0.478 | 0.509 | 0.505 | 0.307 | 0.591 | 0.527 | 0.572 | 0.478 |
| Vitality | 0.618 | 0.147 | 0.665 | 0.666 | 0.684 | 0.368 | 0.672 | 0.672 | 0.739 | 0.694 |
| Social functioning | 0.638 | 0.138 | 0.649 | 0.664 | 0.675 | 0.400 | 0.703 | 0.726 | 0.771 | 0.696 |
| Role-emotional | 0.483 | 0.159 | 0.446 | 0.483 | 0.475 | 0.361 | 0.503 | 0.495 | 0.557 | 0.515 |
| Mental health | 0.482 | 0.138 | 0.511 | 0.519 | 0.528 | 0.299 | 0.623 | 0.515 | 0.613 | 0.543 |
Abbreviations: CS, clinical summary; KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living with Heart Failure Questionnaire; OSU, overall summary; OSY, overall symptoms; PL, physical limitation; QoL, quality of life; SB, symptom burden; SE, self-efficacy and knowledge; SF, symptom frequency; SF-36, Short Form 36; SL, social limitation; SS, symptom stability.
The Spearman correlation coefficients between the physical domain of the KCCQ and the other physical measures (i.e. physical measures in other questionnaires, NYHA functional class, and distance covered in the 6-min walk test) were moderate or high (i.e. 0.40–0.81) and slightly greater than those obtained for the physical domain of the MLHFQ (Table 4).
Table 4. Analysis of the Validity of the Kansas City Cardiomyopathy Questionnaire.
| 6MWT distance | NYHA class | SF-36 physical functioning | MLHFQ physical domain | |
| KCCQ physical limitation | 0.625 | −0.405 | 0.811 | −0.772 |
| MLHFQ physical domain | −0.514 | 0.403 | −0.786 |
Spearman Correlation Matrix a for the Physical Dimensions of the Kansas City Cardiomyopathy Questionnaire (KCCQ), the Minnesota Living with Heart Failure Questionnaire 6 (MLHFQ) and the Short Form 36 (SF-36). With Respect to Subjective Functional Capacity (NYHA Class) and Objective Functional Capacity (Distance Covered in the 6MWT).
a All correlations had a P<.001.
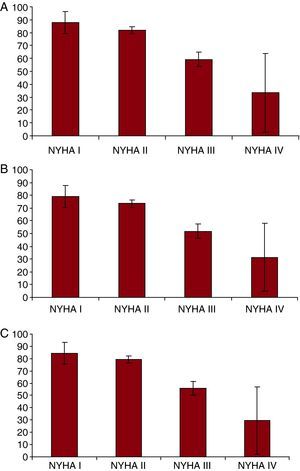
The difference in scores for different NYHA classes was statistically significant (P<.001) for the KCCQ symptoms domain score, overall summary score, and clinical summary score (Figure 1).
Figure 1. Relationship between NYHA Functional Class and KCCQ Symptom Summary Score (panel A), Overall Summary Score (panel B) and Clinical Summary Score (panel C), expressed as means and 95% confidence intervals.P<.001 for the linear trend (Cuzick test) in all three cases.
Reproducibility or ReliabilityThe Cronbach α coefficient (Table 2) was high for all scores, ranging from 0.70 to 0.96 for the KCCQ, 0.75 to 0.94 for the MLHFQ, and 0.74 to 0.93 for the SF-36. The intraclass coefficient (Table 2) was greater than 0.7 for all KCCQ scores, except that the symptom stability score, which was 0.68.
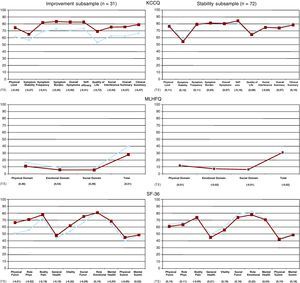
Sensitivity to ChangeThe differences in KCCQ and MLHFQ scores (Figure 2) between the first (visit 1, week 0) and second assessment (visit 2, week 24) in the improvement subsample were statistically significant. However, most scores on the SF-36 did not change significantly. Conversely, there was no significant change in scores on the different questionnaires for the stability subsample of patients who did not change between the two assessments (i.e. no improvement or worsening according to defined clinical criterion).
Figure 2. Change in health-related quality-of-life in two patient subsamples: those who showed improvement (n=31) and those whose condition was stable (n=72) between the first (week 0, blue) and second assessment (week 24, red). Mean scores and effect size coefficients.KCCQ, Kansas City Cardiomyopathy Questionnaire; MLHFQ, Minnesota Living with Heart Failure Questionnaire; SF-36, Short Form 36.
The coefficients for the effect size observed for the three questionnaires are shown in Figure 2. In the analysis of the improvement subsample, the majority of KCCQ and MLHFQ domain and summary scores showed at least moderate values for the coefficient, being greater than 0.4 (i.e. 0.41–0.72 and 0.46–0.54 for the two questionnaires, respectively). The exceptions were the symptom stability item and seven SF-36 scores, all of which had an effect size coefficient less than 0.4. In the stability subsample, the scores on all three questionnaires showed little change, with an effect size coefficient of less than 0.2.
DiscussionIn this multicenter study, we have demonstrated that the Spanish version of the KCCQ functions as a specific Quality-of-Life Questionnaire for patients with CHF. Its metric properties were in line with those of the original version and, in particular, its validity and reliability were excellent.11 In addition, its feasibility for assessing patients was good and it was capable of detecting, at least, moderate changes. These qualities make the Spanish version of the KCCQ an optimal instrument for use in patients with CHF in Spain. In addition, use of the KCCQ enables specific comparisons of HRQL to be made internationally.
With regard to the reliability of the KCCQ, the low percentage of patients who had missing values for scores, which was lower than that observed with the MLHFQ, indicates that the questionnaire was understood and accepted by most CHF patients. The low values for the floor and ceiling effects of the KCCQ and MLHFQ in comparison with the SF-36 point to one of the advantages that specific instruments have over generic questionnaires: they provide a better match with the range of symptom severity experienced by patients.
We have demonstrated that the KCCQ is a valid instrument for measuring HRQL specifically in patients with CHF. The correlations between related domains of the KCCQ, MLHFQ, and SF-36 were high for domains for which a convergent relationship was expected and low for domains for which a discriminatory relationship was expected. Likewise, there were, at least, moderate correlations between KCCQ scores and NYHA functional class and the distance covered in the 6-min walk test.
The significant differences observed in the KCCQ's symptom summary and overall and clinical summary scores demonstrate their capacity to discriminate between different levels of symptom severity. On the other hand, the weak associations observed between domains that are exclusive to the KCCQ (i.e. symptom stability, and self-efficacy and knowledge) and other questionnaire domains indicate that the KCCQ provides added value compared with the MLHFQ at the content level. The range of domains measured by an instrument is a relevant criterion to take into consideration when choosing which instrument to use, whether in clinical practice or in a research setting.
This study showed that the KCCQ has excellent reliability, both with respect to internal consistency and reproducibility, since the coefficients obtained for all scores in the questionnaire were better than those for the standard metrics recommended.33 The Cronbach α for the physical limitation domain and the overall and clinical summary scores exceeded 0.9, which has been proposed as the benchmark for comparisons between individuals. Correspondingly, the intraclass correlation coefficient, which gives a measure of test–retest reliability, was greater than 0.7 for all scores, with values greater than 0.9 for the overall and clinical summary scores. In our study, and in contrast to the original validation of the KCCQ, we did not use the responsiveness statistic used by the authors of the KCCQ to assess sensitivity to change. Rather, we used the Cohen effect size because it is a more accurate method for assessing sensitivity to change.36 Thus, the sensitivity to change observed in the physical limitation domain and in overall and clinical summary scores was moderate35 and similar to that observed for the MLHFQ and for the physical scores of the SF-36.
Study LimitationsThe population selected for the study generally had relatively stable disease (65% were in functional class II) and, therefore, there was little room for improvement. Only 31 patients showed improvement and none showed worsening according to our predefined criterion. The definition of improvement considered in our study remains an indirect assessment of the change in HRQL. On the one hand, the determination of functional class is subject to variability37 and, on the other, although an increase of 6% on the 6-min walk test has been established as clinically significant,8,26,28,29,30 correlations between the distance covered on the test and HRQL scores were often only moderate. Despite using broad inclusion criteria, the patients included cannot be considered completely representative of the whole population of CHF patients given the exclusion criteria inherent in all clinical research of this type.
ConclusionsThe KCCQ is a specific HRQL questionnaire for patients with CHF. It demonstrated good reliability, validity, and sensitivity to change. In addition, bearing in mind that it covers factors not assessed by previous questionnaires specific to CHF, it should be considered for use as an instrument for monitoring patients’ HRQL, either in clinical practice or in a research setting.
FundingFunded by an unconditional grant from Menarini S.A. Specific Action 2008 (PM08 003) of the CIBER for Epidemiology and Public Health.
Conflicts of InterestThe authors state that they have no conflicts of interest.
Investigators of the Valic-KC Study GroupJ. Comin, J. Bruguera, M. Rizzo, N. Manito, J. Lupon, A. Bayés-Genís, Eulalia Roig, M. Crespo-Leiro, C. Naya, F. Ridocci, L. de la Fuente, J.M. González-Matas, P. Pabón, A. Lara, J.L. Manzano, P. Conthe, M. Méndez, L. Pulpón, M. Gómez-Bueno, M. Sanz, T. Blasco, M. Martinez, I. González, G. Guzman, A. Llácer, K. Montes, A. Salcedo, J. Zumalde, N. Murga, J.V. Climent, M. Anguita, J.M. García, J. Beltrán, L. Pastor, A. Castro, P. Gallego, F. Sabatel, E. Sanchez, J.R. González-Juanatey, A. Varela, D. Pascual, P. Valdovinos, J. Quiles B. Sevilla, D. Jiménez, J. Noval, B. Fuertes, N. Tarín, R. Elosua, M. Cabañero, J. Vila, M. Ferrer, O. Garín, A. Pont.
Received 21 January 2010
Accepted 21 July 2010
Corresponding author. Programa de Insuficiencia Cardiaca, Servicio de Cardiología, Hospital del Mar (Parc de Salut Mar), Paseo Marítimo, 25-29, 08003 Barcelona, Spain. jcomin@hospitaldelmar.cat