Although atherogenic dyslipidemia is a recognized cardiovascular risk factor, it is often underassessed and thus undertreated and poorly controlled in clinical practice. The objective of this study was to reach a multidisciplinary consensus for the establishment of a set of clinical recommendations on atherogenic dyslipidemia to optimize its prevention, early detection, diagnostic evaluation, therapeutic approach, and follow-up.
MethodsAfter a review of the scientific evidence, a scientific committee formulated 87 recommendations related to atherogenic dyslipidemia, which were grouped into 5 subject areas: general concepts (10 items), impact and epidemiology (4 items), cardiovascular risk (32 items), detection and diagnosis (19 items), and treatment (22 items). A 2-round modified Delphi method was conducted to compare the opinions of a panel of 65 specialists in cardiology (23%), endocrinology (24.6%), family medicine (27.7%), and internal medicine (24.6%) on these issues.
ResultsAfter the first round, the panel reached consensus on 65 of the 87 items discussed, and agreed on 76 items by the end of the second round. Insufficient consensus was reached on 3 items related to the detection and diagnosis of atherogenic dyslipidemia and 3 items related to the therapeutic goals to be achieved in these patients.
ConclusionsThe external assessment conducted by experts on atherogenic dyslipidemia showed a high level of professional agreement with the proposed clinical recommendations. These recommendations represent a useful tool for improving the clinical management of patients with atherogenic dyslipidemia. A detailed analysis of the current scientific evidence is required for those statements that eluded consensus.
Keywords
In recent years, the treatment of hypercholesterolemia has become a cornerstone of the primary and secondary prevention of cardiovascular disease. The recent European guidelines on cardiovascular disease prevention,1 which incorporate the document on cholesterol management agreed upon by the European Society of Cardiology and the European Atherosclerosis Society,2 focuses on the need to improve the percentage of patients reaching the therapeutic targets of low-density lipoprotein cholesterol (LDL-C). This report recognizes both triglycerides and high-density lipoprotein cholesterol (HDL-C) as independent risk factors, and stresses the importance of the latter lipid fraction in the estimation of total cardiovascular risk; nonetheless, HDL-C is not a recommended therapeutic target.
The metabolic abnormality atherogenic dyslipidemia is characterized by hypertriglyceridemia, a reduction in HDL-C concentrations, and the presence of small, dense LDL particles. This dyslipidemia is common in patients with coronary heart disease, metabolic syndrome, and type 2 diabetes mellitus (DM), and is largely the cause of lipid-related residual vascular risk.3,4 Although its prevalence could be expected to increase in parallel with that of DM and obesity, atherogenic dyslipidemia is largely underestimated and, consequently, undertreated in clinical practice. Accordingly, the present study attempts to promote and develop a multidisciplinary consensus on atherogenic dyslipidemia by integrating the best available evidence and the experience of a broad panel of professionals from different medical specialties. We aimed to establish a set of criteria and clinical recommendations on atherogenic dyslipidemia to optimize the prevention, early detection, diagnostic evaluation, therapeutic approach, and clinical follow-up of atherogenic dyslipidemia in the distinct medical settings of the health care system.
METHODSStudy DesignA modified Delphi method5 was used to obtain the best possible agreement among an extensive panel of medical experts in dyslipidemia. This method involves a structured technique for reaching consensus among remotely located professionals, and is a variant of the original procedure developed by Dalkey et al. in Rand Corporation Santa Monica (California, United States)6,7 that maintains its principal advantages (controlled interaction among panel members, the opportunity to reflect and reconsider opinions without loss of anonymity, and statistical validation of the consensus reached) compared with other alternative techniques, and resolves some of its main disadvantages (biases).8
The modified Delphi method requires successive rounds of a structured e-mailed survey. Between both rounds of responses, the expert panelists receive feedback from the intermediate results so that they can confidentially contrast their personal opinions with those of the other participants and, if necessary, reconsider their initial opinions on the statements without consensus.
The study was carried out in 4 phases: a) formation of a scientific committee, responsible for the selection of the experts panel and formulation of the survey items; b) creation of an expert panel of professionals from 4 medical specialties (cardiology, endocrinology, internal medicine, and family and community medicine), with special interest and experience in the field of dyslipidemias, with the sole task of completing the questionnaire; c) 2 rounds of e-mail surveys with intermediate processing of the opinions and delivery of feedback to the panelists, and d) collection, analysis of results, and discussion of the conclusions in a face-to-face session of the scientific committee.
Questionnaire DevelopmentThe authors of the current paper (internists, endocrinologists, and family physicians) formed the scientific committee behind the project due to their extensive expertise and professional experience in this field. Together with the collaboration of an external methodology consultant (the medical director of the company that supported the project), the committee developed the content of the Delphi questionnaire. For survey development, a literature search was carried out that prioritized the identification of systematic reviews and other types of critical summaries of the scientific literature through consultation of the usual bibliographic databases (MEDLINE, EMBASE, and the Índice Médico Español [Spanish Medical Index]),9 as well as a manual review of the bibliographic references obtained to locate other articles that could be of interest, through keywords such as atherogenic dyslipidemia and cardiovascular risk.
Each item of the survey evaluated by the panel was drafted by bearing in mind that it was a statement–positive or negative–expressing either a professional opinion or a clinical recommendation, that responded to clinical doubts or aspects of interest or controversy in the clinical management of patients with atherogenic dyslipidemia. The final version of the questionnaire included 87 items (Table), grouped in the following subject areas: general concepts (10 items), impact and epidemiology (4 items), cardiovascular risk (32 items), detection and diagnosis (19 items), and treatment (22 items).
Results of the Level of Agreement Achieved by the Experts After the 2 Rounds
| Median | Panelists in favor, % | Mean | ||
| I. Concept | ||||
| 1. | Atherogenic dyslipidemia is an alteration of lipid metabolism that is related to other aspects of metabolism such as obesity, metabolic syndrome, and insulin resistance | 9 | 100 | 8.58 |
| 2. | Atherogenic dyslipidemia is characterized by the combination of low HDL-C, elevated triglycerides, and a high proportion of small, dense LDL particles | 9 | 98.5 | 8.58 |
| 3. | The presence of a high proportion of small, dense LDL particles has been called the “atherogenic lipoprotein phenotype” | 8 | 84.6 | 7.86 |
| 4. | The higher the concentration of triglycerides, the greater the proportion of small, dense LDL particles | 8 | 68.7 | 7.19 |
| 5. | Atherogenic dyslipidemia is related to adiposity | 7 | 70.8 | 7.05 |
| The cardiovascular benefits dependent on HDL-C are related to: | ||||
| 6. | Reverse cholesterol transport from the periphery | 9 | 93.8 | 8.31 |
| 7. | The vasodilatory effect | 5* | 68.0 | 5.34 |
| 8. | The antioxidant effect | 7 | 70.8 | 7.12 |
| 9. | The antithrombotic effect | 6* | 42.2 | 5.98 |
| 10. | The antiinflammatory effect | 7 | 75.0 | 6.91 |
| II. Impact and epidemiology | ||||
| 11. | Atherogenic dyslipidemia is a lipoprotein phenotype frequently and particularly prevalent in patients with a cardiovascular event | 8 | 84.6 | 7.32 |
| 12. | Atherogenic dyslipidemia is the characteristic lipid alteration of type 2 DM and metabolic syndrome | 9 | 98.5 | 8.35 |
| 13. | Atherogenic dyslipidemia is often associated with obesity | 8 | 93.7 | 7.97 |
| 14. | The main cause of elevated residual vascular risk is the presence of atherogenic dyslipidemia | 7 | 79.7 | 7.19 |
| III. Associated CVR | ||||
| 15. | Clinical evidence shows that atherogenic dyslipidemia is an important CVR factor | 8 | 95.3 | 8.09 |
| 16. | The risk attributable to atherogenic dyslipidemia is independent and additional to that related to LDL-C | 8 | 81.5 | 7.48 |
| 17. | Atherogenic dyslipidemia is associated with a CVR burden similar to that associated with hypercholesterolemia | 7 | 67.4 | 5.96 |
| 18. | The presence of atherogenic dyslipidemia increases CVR, even with “normal” LDL-C | 8 | 87.5 | 7.70 |
| The increase in CVR in an atherogenic dyslipidemia patient is associated with: | ||||
| 19. | An increase in triglycerides | 7 | 72.9 | 6.60 |
| 20. | A decrease in HDL-C | 8 | 85.5 | 7.77 |
| 21. | The phenotype of small, dense LDL particles | 8 | 95.1 | 8.06 |
| Triglycerides: | ||||
| 22. | Are an independent risk factor | 7* | 62.5 | 6.44 |
| 23. | Are treatable factors | 8 | 84.1 | 7.48 |
| 24. | The increase in risk attributable to hypertriglyceridemia varies between 60% and 75% | 5* | 38.3 | 4.81 |
| 25. | Postprandial triglycerides have a higher predictive value for cardiovascular disease than fasting triglycerides | 7 | 81.2 | 7.06 |
| 26. | The role of triglycerides as a CVR factor is dependent on the HDL-C concentration | 7 | 81.2 | 6.81 |
| 27. | The combination of low HDL-C and high triglycerides has a synergistic action with CVR | 8 | 87.5 | 7.72 |
| HDL-C: | ||||
| 28. | Is an independent predictor of cardiovascular disease | 8 | 89.2 | 8.05 |
| 29. | Should be considered in dyslipidemia management | 8 | 92.3 | 8.15 |
| 30. | HDL-C concentration predicts the risk of coronary heart disease with any LDL-C level, even the lowest | 8 | 87.7 | 7.74 |
| 31. | CVR in patients with normal LDL-C and low HDL-C is higher than in those with high LDL-C and high HDL-C | 7 | 67.3 | 6.73 |
| 32. | It has been shown that the benefit is greater (a 2%-3% reduction in coronary risk) if HDL-C increases by 1% than if LDL-C decreases by 1% (a 1% reduction in risk) | 7 | 68.9 | 6.74 |
| 33. | By considering all intervention studies available, a decrease of 1 mmol/L in LDL-C achieves at most a 23% reduction in coronary events | 8 | 85.7 | 7.48 |
| 34. | Three-quarters of patients with a history of cardiovascular disease under statin treatment die from coronary events, which suggests that other factors that maintain this residual risk can be treatable | 8 | 84.1 | 7.46 |
| 35. | In patients treated with statins, even at high doses, a high residual risk remains, which is partly lipid-related and is susceptible to additional therapeutic intervention | 8 | 88.7 | 7.76 |
| 36. | The residual risk associated with high triglycerides or low HDL-C is not eliminated with statins | 7 | 84.4 | 7.28 |
| Despite optimal statin treatment, even with a maximal reduction in LDL-C, a significant residual risk remains in patients: | ||||
| 37. | In primary prevention | 7* | 58.3 | 6.29 |
| 38. | In secondary prevention | 8 | 95.3 | 8.06 |
| 39. | At high risk | 8 | 90.6 | 7.88 |
| 40. | Treated intensively | 8 | 82.5 | 7.41 |
| 41. | Atherogenic dyslipidemia is the main component of lipid-related residual risk | 8 | 87.1 | 7.90 |
| The LDL-C-independent CVR that is related to atherogenic dyslipidemia is particularly important in patients with: | ||||
| 42. | Type 2 DM | 8 | 98.5 | 8.32 |
| 43. | Metabolic syndrome | 8 | 96.9 | 8.23 |
| 44. | Obesity | 8 | 80.0 | 7.45 |
| 45. | In diabetic patients with high triglycerides, non-HDL cholesterol must be determined to evaluate CVR | 8 | 84.4 | 7.72 |
| 46. | In patients with atherogenic dyslipidemia, SCORE tables are not useful for calculating CVR in primary prevention because they do not take full account of the risk associated with atherogenic dyslipidemia | 7 | 76.6 | 7.17 |
| IV. Detection and diagnosis | ||||
| 47. | The hypertriglyceridemic waist helps to identify those patients with atherogenic dyslipidemia | 7 | 79.4 | 7.27 |
| To diagnose atherogenic dyslipidemia, it is sufficient to measure: | ||||
| 48. | Only HDL-C | 3 | 67.2 | 3.23 |
| 49. | Only triglycerides | 3 | 73.8 | 2.93 |
| 50. | HDL-C and triglycerides | 8 | 93.8 | 7.83 |
| To evaluate atherogenic dyslipidemia, a sufficient diagnostic test is determination of: | ||||
| 51. | ApoB | 3* | 57.1 | 4.39 |
| 52. | Non-HDL cholesterol | 7* | 55.6 | 5.53 |
| 53. | Both apoB and non-HDL cholesterol; these diagnostic tests are sufficient for the evaluation of atherogenic dyslipidemia because they are equivalent | 7* | 53.2 | 5.85 |
| Small, dense LDL is a component of atherogenic dyslipidemia that is reflected by the level of: | ||||
| 54. | ApoB | 7 | 76.2 | 7.10 |
| 55. | Non-HDL cholesterol | 7 | 71.7 | 6.33 |
| Targets and control | ||||
| In the presence of atherogenic dyslipidemia, the primary control target is: | ||||
| 56. | LDL-C | 7 | 67.7 | 6.97 |
| 57. | Non-HDL cholesterol | 7 | 77.6 | 6.59 |
| 58. | Overall control of the lipid profile | 8 | 80.6 | 7.68 |
| 59. | In patients at high vascular risk with atherogenic dyslipidemia and LDL-C target values, it is more effective to correct the atherogenic dyslipidemia than to try to further reduce LDL-C | 7 | 73.4 | 6.88 |
| 60. | The therapeutic targets for HDL-C and triglycerides are similar in primary and secondary prevention | 7* | 67.3 | 5.76 |
| 61. | In high-risk patients, the secondary therapeutic targets are:• Triglycerides<150 mg/dL• HDL-C>40 mg/dL (men) or >50 mg/dL (women) | 8 | 96.8 | 8.21 |
| The algorithm of targets to be achieved in atherogenic dyslipidemia is: | ||||
| 62. | Reduce triglycerides and then try to increase HDL-C | 7* | 54.2 | 5.85 |
| 63. | Increase HDL-C and then try to reduce triglycerides | 5* | 41.3 | 4.93 |
| 64. | The frequency of monitoring and follow-up of patients with atherogenic dyslipidemia treated to reduce their components should be similar to that used when trying to control LDL-C | 8 | 78.1 | 7.14 |
| 65. | All patients with hard-to-control atherogenic dyslipidemia must be referred to the lipid clinic | 7 | 72.9 | 6.81 |
| V. Treatment | ||||
| 66. | The overall control of the lipid profile in patient with atherogenic dyslipidemia fairly frequently requires combination lipid-lowering therapy | 8 | 93.8 | 7.92 |
| 67. | There is ample scientific evidence of the benefit of treating atherogenic dyslipidemia in the prevention of cardiovascular diseases | 7 | 73.4 | 6.97 |
| 68. | An increase in HDL-C with drugs or with lifestyle changes has been associated with a decrease in CVR | 8 | 76.6 | 7.09 |
| 69. | In patients with somewhat elevated LDL-C, HDL-C deficiency, and high triglycerides, the treatment of choice is a statin | 8 | 80.0 | 7.28 |
| 70. | In patients with somewhat elevated LDL-C, HDL-C deficiency, and high triglycerides who fail to achieve overall control of the lipid profile with a statin, the combination of a statin+fibrate is a good therapeutic option | 8 | 87.3 | 7.60 |
| 71. | The fenofibrate dose must be adjusted in patients with mild or moderate renal failure (stages 2 or 3) and fibrates must be avoided in the more advanced stages of this disease | 8 | 81.5 | 7.38 |
| 72. | Due to its lower potential for interactions and the ample evidence derived from routine clinical use and clinical studies, fenofibrate is the fibrate of choice for combination with statins | 9 | 98.4 | 8.44 |
| 73. | Fibrate treatment in patients with type 2 DM decreases both macrovascular and microvascular complications | 7 | 67.7 | 6.84 |
| 74. | The preventative effect of fibrates against coronary events is influenced by the baseline HDL-C and triglyceride concentrations | 8 | 87.1 | 7.55 |
| 75. | Gemfibrozil is the fibrate with the greatest potential for interactions and its combination with statins is contraindicated | 8 | 87.3 | 7.89 |
| 76. | The ACCORD study results have shown that treatment of atherogenic dyslipidemia in diabetic patients has cardiovascular prevention benefits | 8 | 76.6 | 7.22 |
| 77. | Fenofibrate treatment reduces CVR in patients with atherogenic dyslipidemia | 7 | 83.1 | 7.23 |
| 78. | The most effective drugs for reducing triglycerides are fibrates, followed by nicotinic acid, omega-3 fatty acids, and statins | 8 | 85.9 | 7.64 |
| 79. | Lifestyle changes, such as diet and physical activity, are a fundamental component of the treatment of patients with atherogenic dyslipidemia | 9 | 98.7 | 8.50 |
| The most effective drugs for treating HDL-C deficiency are: | ||||
| 80. | Fibrates | 7 | 75.4 | 6.91 |
| 81. | Statins | 3 | 79.6 | 3.22 |
| 82. | Nicotinic acid | 8 | 93.8 | 7.92 |
| 83. | Omega-3 fatty acids lack a significant effect on HDL-C and their lipid-lowering efficacy is limited to triglycerides | 7 | 71.4 | 6.87 |
| 84. | Nicotinic acid increases HDL-C and decreases triglycerides, but its frequent adverse effects limit its clinical application | 8 | 89.2 | 7.62 |
| 85. | Fibrates act on HDL-C and triglycerides, are generally well tolerated, and have few adverse effects | 8 | 96.9 | 8.00 |
| 86. | In patients with atherogenic dyslipidemia and high CVR whose hypertriglyceridemia or HDL-C deficiency is not controlled with statins + fibrates, triple therapy with nicotinic acid may be necessary | 7 | 80.0 | 7.12 |
| 87. | When triglycerides exceed 500 mg/dL, their reduction is a priority due to the risk of pancreatitis | 8 | 87.5 | 7.92 |
apoB, apolipoprotein B; CVR, cardiovascular risk; DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
“Panelists in favor” is the percentage of panelists that scored within the 3-point range that contained the median (1-3, 4-6, 7-9).
A single rating scale was proposed for all statements, namely an ordinal 9-point Likert-type scale (1, complete disagreement; 9, complete agreement), according to the format developed in the UCLA-Rand Corporation for evaluating the appropriate use of health care technology.9 The response categories were described through linguistic qualifiers in 3 ranges (1-3, disagreement; 4-6, neither agreement not disagreement; 7-9, agreement). In each case, survey respondents could detail their personal opinions and choose between the 3 points contained in each range. Statements that were unanswered because the panelists considered themselves inadequately qualified in the area were treated as lost cases for statistical purposes.
The method used permits and encourages the confidential exchange of comments and opinions, clarifying personal stances. Thus, the survey allows free observations to be added to each item, as well as a final section for new proposals to be evaluated by the committee.
Selection of the Expert PanelThe panel experts were selected by the scientific committee on the basis of the following criteria: that they be representatives of their clinical collective that make regular decisions about the disease being studied, professionally recognized for their experience and scientific opinion (leadership in the field), and have a special interest in the field of dyslipidemias. For their identification, a snowball sampling strategy was used, beginning with the personal contacts of the members of the committee, who in turn proposed new candidates who were leaders in their professional field.10 After this process, 81 professionals were invited by letter to participate. Of these, 65 experts from all the autonomous regions of Spain agreed to participate. All of the participants were practicing physicians: 24.6% were endocrinologists; 23.0% were cardiologists; 27.7% were primary care physicians, and 24.6% were internists. The project field work was carried out in a 6-week period in February and March 2012 through the use of e-mail to distribute and collect the forms.
Analysis and Interpretation of the ResultsThe median position of the group scores and the level of agreement between these scores were used to analyze the group opinion for each statement.11 It was accepted that there was agreement when less than a third of the experts scored outside the 3-point range (1-3, 4-6, 7-9) containing the median; there was disagreement when the scores of a third or more of the panelists were in the 1-3 range, and another third or more in the 7-9 range; the remaining cases, showing neither agreement nor disagreement, were considered to have indeterminate agreement. When there was agreement, the type of group consensus was determined by the median value: majority agreement with the item if the median was greater than or equal to 7 general disagreement with the item if the median was lower than or equal to 3 uncertain items for a representative majority of the group if the median was located in the 4-6 range.
All items showing a lack of a clear consensus for or against the statement posed—that is, the items showing disagreement or indeterminate or uncertain consensus—were put forward for reconsideration by the panel in the second Delphi round. In addition, those items with a wide spread of opinions among the survey respondents (interquartile range greater than or equal to 4 points) also underwent reassessment. Between both rounds, the panelists were informed of the detailed distribution of the responses of the group in the first survey through bar graphs to facilitate comments and clarifications from each participant. After the second round, identical criteria were applied to discriminate between those items definitely agreed upon and those in which it was impossible to unify the opinions of the panel.
To enable comparisons among items, the mean scores of the panelists were calculated for each statement. The more extreme the mean score of an item (closer to 1 or 9), the clearer the consensus reached, either in disagreement or agreement, respectively, on the view expressed by each item.
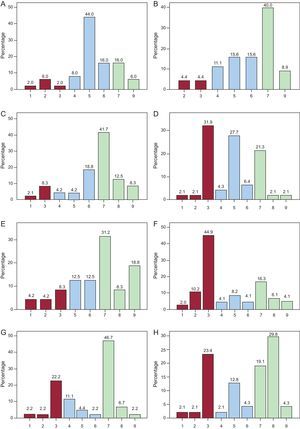
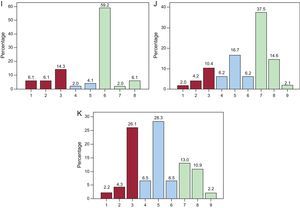
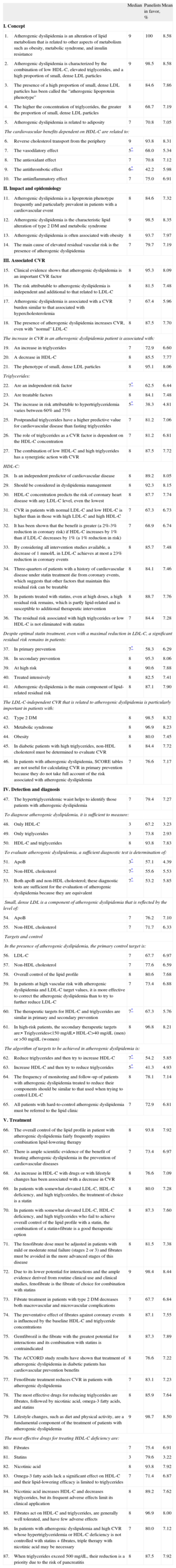
RESULTSThe 65 experts consulted completed the 2 evaluation rounds without proposing new items. In the first round, agreement was reached on 65 of the 87 statements analyzed according to the evaluation criteria established; 63 of these items were in group agreement and 2 were in group disagreement. Of the 22 remaining items that were returned for reconsideration by the experts, agreement was reached for 11 (10 in group agreement and only 1 in general disagreement) in the second round. Overall, the panel achieved sufficient consensus in 87.4% of the proposed statements, and an uncertain consensus persisted in 11 items after the 2 rounds. For each item, we have detailed the central tendencies (median and mean scores) and the percentage of panelists that gave a score within the 3-point range that contained the median (1-3, 4-6, 7-9) (Table). The distribution of the scores awarded by the panelists to the statements eluding consensus is also shown (Figure).
Distribution of expert opinions on the items lacking agreement. A: vasodilatory effect of high-density lipoprotein cholesterol. B: antithrombotic effect of high-density lipoprotein cholesterol. C: triglycerides are an independent risk factor. D: the increase in risk attributable to hypertriglyceridemia varies between 60% and 75%. E: persistence of residual risk despite optimal treatment with statins in patients in primary prevention. F: to evaluate atherogenic dyslipidemia, apolipoprotein B measurement is a sufficient diagnostic test. G: to evaluate atherogenic dyslipidemia, non-high-density lipoprotein cholesterol measurement is a sufficient diagnostic test. H: to evaluate atherogenic dyslipidemia, apolipoprotein B and non-high-density lipoprotein cholesterol determinations are sufficient diagnostic tests. I: the therapeutic targets for high-density lipoprotein cholesterol and triglycerides are similar in primary and secondary prevention. J: the algorithm for the targets to be achieved in atherogenic dyslipidemia is triglyceride reduction followed by an increase in high-density lipoprotein cholesterol. K: the algorithm for the targets to be achieved in atherogenic dyslipidemia is an increase high-density lipoprotein cholesterol followed by a reduction in triglycerides.
In general, the opinion of the various participating specialists on the criteria and recommendations for the clinical management of atherogenic dyslipidemia was largely uniform, with a significant degree of consensus reached in most (76 of the 87) of the items.
The level of agreement on the characterization of atherogenic dyslipidemia is notable, although there were disagreements in the lipid markers most specifically identifying this disorder. Thus, all of the experts recognized atherogenic dyslipidemia as an alteration directly related to metabolic disorders, such as type 2 DM, metabolic syndrome, obesity, and/or insulin resistance, that can be clearly identified phenotypically by an increase in triglycerides, a decrease in HDL-C, and the presence of small, dense LDL particles.
In agreement with the best scientific evidence,12 the consensus in the main proposals that associated atherogenic dyslipidemia with an elevated cardiovascular risk was striking, with two-thirds of those polled believing that its impact is similar to that of hypercholesterolemia. About 80% of the experts considered atherogenic dyslipidemia to be an important component of residual risk, and 85% of those consulted recognized that a decrease in LDL-C achieves a mere 23% reduction in coronary events. Moreover, the prominence of atherogenic dyslipidemia in residual risk is unanimously recognized in disorders such DM and metabolic syndrome.
Despite the evidence linking both triglycerides and HDL-C with an elevated risk,12–17 a certain skepticism remains, as well as doubts about the role played by each of these lipid components. These uncertainties affect the choice of targets. Notably, two-thirds of the panelists indicated that low HDL-C concentrations with normal LDL-C levels carries a greater risk than elevated LDL-C levels with normal or elevated HDL-C concentrations, which concurs with the available scientific evidence.18,19 Consequently, 67% of the experts identified HDL-C as a therapeutic target independent of LDL-C, although the agreement was restricted to those high-risk patients, including those with atherogenic dyslipidemia, that require control of the entire lipid profile. In these high-risk patients, the therapeutic target was clear—triglycerides below 150mg/dL and cholesterol greater than 40/50mg/dL (men/women)—which is in accordance with international recommendations.1,2
Finally, with modest variations, the section on treatment showed the best agreement: each and every one of the proposals was agreed upon by the majority. Thus, more than 90% of the experts were of the opinion that, although the primary therapeutic target continues to be LDL-C, in the presence of atherogenic dyslipidemia, combined drug treatment can be an option to reduce residual risk,4,20 and that the best therapeutic option is the combination of a statin with fenofibrate (98% of the experts), due to the triglyceride-lowering effect of fibrates and contraindication against using gemfibrozil with statins. Furthermore, the validity of this approach was recently confirmed in a meta-analysis of the effects of fenofibrate.21 This consensus on the need for combined treatment for selected populations with atherogenic dyslipidemia is as widespread among experts as the consistent recommendation that lifestyle modification is a key strategy in these patients.12
Two findings are worthy of consideration. First, a small but significant 25%-30% of the respondents harbored doubts about the clinical benefit of combined statins and fenofibrates on cardiovascular prevention in diabetics, indicating that extra effort is required to clarify that diabetic patients with atherogenic dyslipidemia can benefit from this treatment.22,23 Second, regarding the treatment of low HDL-C syndrome, although 75% of those consulted indicated that fibrates are the drug of choice, 93% selected nicotinic acid, with 90% recognizing that its use is limited due to adverse effects.
On the other hand, the statements that were the source of disagreement among the experts centered on the following topics: the existence of cardiovascular benefits of HDL-C that are separate from their effect on reverse cholesterol transport, analytical recognition of atherogenic dyslipidemia through markers other than triglyceride and HDL-C concentrations, the role of hypertriglyceridemia alone as a risk factor for and its impact on cardiovascular risk, the need to tackle the residual risk that is not dependent on LDL-C in those patients that are in primary prevention, and the therapeutic targets to consider and the approach necessary. Notably, some of the statements that showed a lack of consensus (such as the vasodilatory or antithrombotic effect of HDL) are well demonstrated, such as the antiatherogenic effects of the HDL molecule.12,24 Regarding the diagnostic markers of atherogenic dyslipidemia, more than half of those surveyed considered that apolipoprotein B and/or non-HDL cholesterol were adequate markers, but the median and mean only showed uncertain agreement due to the wide variety of opinions in the answers.
As an isolated risk factor, a decrease in HDL-C was better known than isolated hypertriglyceridemia. Although the scientific evidence indicates an association between triglyceride-rich lipoproteins and cardiovascular risk, with a variable associated burden according to epidemiological studies,13–15 only 62.5% of experts consulted accepted that isolated hypertriglyceridemia was an independent risk factor, and a lower percentage considered that hypertriglyceridemia was accompanied by a marked increase in cardiovascular risk.
Statins alone are unable to eliminate the cardiovascular risk attributable to atherogenic dyslipidemia.4 Accordingly, the therapeutic strategy in this situation in both primary and secondary prevention must include a reduction in triglycerides and an increase in HDL-C, as well as LDL-C control. However, although this viewpoint appeared to be clear in this study for secondary prevention, only about 60% of the experts thought the same for patients in primary prevention, which still leaves this topic open to question, given that only 70% believed that the targets for triglycerides or HDL should be the same regardless of circumstance.
Therefore, our results indicate a broad consensus in the majority of the features of atherogenic dyslipidemia dealt with by the survey statements: epidemiology, associated vascular risk, detection and diagnosis, and therapeutic management. However, some aspects (a minority) that eluded a majority consensus among the experts remain, including certain pathophysiological features of atherogenic dyslipidemia and the eventual differences in therapeutic approach, which depend on whether the patient is in primary or secondary prevention.
CONCLUSIONSConsequently, and as one of the first conclusions, it should be noted that the experts agreed that additional efforts be made in the diagnosis of atherogenic dyslipidemia due to its associated risk, first and foremost in high-risk populations such as patients in secondary prevention with type 2 DM or metabolic syndrome, a patient group that shows a high prevalence of this disorder. Accordingly, additional therapeutic efforts may be required in these patients to control atherogenic dyslipidemia, via combined drug therapy if necessary.
The apparent disparities among the available guidelines and the opinions expressed by the experts on the importance of treatment may be influenced by the focus of guidelines on LDL-C as a risk factor (“LDL-centric”). In contrast, the participants, although also attentive to the guidelines, had a special sensitivity toward other forms of dyslipidemia.
In conclusion, the high degree of consensus of the expert panel on the different aspects of atherogenic dyslipidemia suggests that the majority of the criteria, evidence, and clinical recommendations formulated reflect the professional opinion of most Spanish specialists. Thus, there is some guarantee on the practical use of the knowledge and a lower variability in the clinical management of patients with atherogenic dyslipidemia.
Nonetheless, the items showing clear disagreement among the experts or even an ambiguous situation compel the development of future approaches. Specifically, this situation invites reflection on the relevance of an exhaustive search of the scientific evidence on the items lacking agreement among the majority of the experts. Moreover, it is recommended that studies be developed in Spain to resolve doubts and standardize professional opinion on certain aspects of diagnosis or treatment, such as the effectiveness of combined statin plus fenofibrate treatment in high-risk patients with atherogenic dyslipidemia.
FUNDINGAbbott España has provided support to the scientific committee in developing the field work of the Delphi survey of this study, without participating in the design and data analysis or in the writing of the present article.
CONFLICTS OF INTERESTNone declared.
To the survey panelists, for their expert contribution to the Delphi survey (Appendix). To Luzán 5 (Madrid), the company in charge of carrying out the project, for providing assistance to the scientific committee in the tasks of project design, statistical analysis, and obtaining the final results.
| Primary care | Artemio Álvarez Cosmea, César Asenjo Vázquez, Juan P. Benítez Ortiz, Mariano Blasco Valle, Carlos Brotons Cuixart, Serafín de Abajo Olea, Isabel Egocheaga Cabello, Rosaura Figueras Camós, Josep Franch Nadal, Francisco J. García-Norro Herreros, Luis García Ortiz, Olga García Vallejo, Javier García Soidán, Isidro López Rodríguez, Antonio Maiques Galán, Vicente Pascual Fuster, Adalberto Serrano Cumplido, Manuel Taboada Taboada, José Vicente Lozano |
| Cardiology | Vicente Bertomeu González, Jesús M. de la Hera Galarza, Alfonso del Río Ligorit, Jesús Egido de los Ríos, Antonio Esteban Luque, Antonio García Quintana, José S. Hevia Navas, Víctor López García-Aranda, Juan P. López Ramírez, Irene Marín Marín, Javier Mora Robles, Gerardo Moreno Terribas, Ignacio Plaza Pérez, César Romero Menor, Pedro J. Serrano Aísa, José L. Zamorano Gómez |
| Endocrinology | Jordi Anglada Barceló, Juan F. Ascaso Gimilio, Antonio Becerra Fernández, Diego Bellido Guerrero, Ignasi Conget Donlo, Fernando Escobar Jiménez, Luis F. Pallardo Sánchez, Concepción García Calzado, Luis Irigoyen Cucalon, Juan A. Paniagua González, Carlos Pesquera González, José T. Real Collado, Francisco J. Tébar Massó, José I. Vidal Pardo, Lluis Vila Ballester |
| Internal medicine | Fátima Almagro Múgica, Aurelio Baixauli Rubio, Jesús Egido de los Ríos, Jacinto Fernández Pardo, Juan C. Ferrando Vela, Jesús Galiana Gómez, Diego Godoy Rocati, Carlos Guijarro Herráiz, José L. Hernández Hernández, Carlos Lahoz Rallo, José López Miranda, Alipio Mangas Rojas, Lluís Masana Marín, Francisco Pérez Jiménez, Pedro Sáenz Aranzubia, Manuel Suárez Tembra, Alberto Zamora Cervantes |