Keywords
INTRODUCTION
Stress hyperglycemia is a common finding in patients with acute myocardial infarction (AMI), which has been recognized as an acute metabolic stress. Several studies have shown that hyperglycemia on admission in acute coronary syndrome (ACS) patients is common, being associated with an increased risk of death and inhospital morbidity.1-11
Although most studies have focused their attention on the prognostic value of blood glucose at admission, the assessment of blood glucose levels during hospitalization is gaining a growing importance. However, little is known about the prognostic value of the magnitude of glycemia variation during an ACS.
The aim of this study was to evaluate the impact of magnitude of glycemia variation during hospitalization on short and long-term prognosis, in 2 ACS populations—patients with and without previous diagnosis of diabetes—, and to identify independent predictors of post-discharge endpoints in these populations.
METHODS
We performed a retrospective analysis of a database including 1210 consecutive patients admitted to a single coronary care unit for ACS between May 2004 and July 2007.
ACS was defined according to current guidelines as unstable angina, non-ST elevation (NSTEMI), and ST elevation myocardial infarction (STEMI).12,13
Casual (non-fasting) plasma glucose was measured at hospital admission and magnitude of glycemia variation was defined as the difference between admission glycemia and the lowest fasting glycemia during hospitalization.
Our population was divided in 2 subpopulations: patients with previous diagnosis of diabetes (n=386) and nondiabetics (n=824). Each of these subpopulations was divided in 4 groups, according to the quartiles of glycemia variation: diabetics (Q1: <46 mg/dL; Q2: 46-88 mg/dL; Q3: 88-164 mg/dL; Q4: ≥164 mg/dL) and nondiabetics (Q1: <14 mg/dL; Q2: 14-30 mg/dL; Q3: 30-60 mg/dL; Q4: ≥60 mg/dL).
Patients were classified as having previous diabetes if they had been given that diagnosis in the past, if their medical records contained documentation of a previous history of diabetes or if the patient was treated with an oral antidiabetic agent or insulin at the time of hospital admission.
Demographic data, cardiovascular risk factors, comorbidities, and drug treatment before and during hospital stay as well as at discharge were collected. Furthermore, laboratory parameters, including first blood glucose upon admission, fasting glycemia, glycated hemoglobin (HbA1c), myocardium necrosis, inflammatory and renal function markers, hemoglobin, lipid profile, ECG data, and left ventricular ejection fraction evaluated by echocardiography, were also determined.
The use and mode of reperfusion (thrombolysis, percutaneous coronary intervention, and/ or coronary artery bypass grafting) was also documented. Moreover, in-hospital morbidity data, including ventricular fibrillation, cardiogenic shock, cardiac arrest, recurrent myocardial infarction, and pulmonary edema, were recorded and globally assessed.
Patients were followed during an average of 18 months after ACS, by review of medical records and telephone interview. Thirty-three patients (2.7% of total population) were lost to follow-up. Readmission for ACS or worsening heart failure, non-programmed revascularization, and death were considered as post-discharge endpoints.
Analysis of group differences was performed using the Kruskall-Wallis test (median [P25-P75]) for continuous variables and c2 test for trend for categorical variables. A P value less than .05 was considered statistically significant. Multivariate logistic regression analysis was then performed to determine independent predictors of post-discharge endpoints. In the multivariate logistic regression analysis model, clinically relevant variables were tested: age, smoking habits, previous treatment with antiplatelet agents and angiotensin-converting enzyme inhibitor (ACE inhibitors), Killip class, systolic and diastolic blood pressure at admission, heart rate at admission, left ventricular ejection fraction, creatinine clearance, C-reactive protein, and magnitude of glycemia variation.
Kaplan-Meier analysis was used to illustrate follow-up mortality and post-discharge endpoints in the nondiabetic population.
RESULTS
In our population, 20.5% of nondiabetic patients were admitted by unstable angina, 45.5% by NSTEMI, and 34.0% by STEMI, whereas 19.5% of diabetics were admitted by unstable angina, 52.1% by NSTEMI, and 28.4% by STEMI.
Nondiabetic patients with higher magnitude of glycemia variation were older, but there were no significant demographic differences in the diabetic group (Tables 1 and 2).
Risk profile was similar between quartiles. Furthermore, nondiabetic patients in higher quartiles were more often previously treated with diuretics and less treated with beta-blockers and nitrates, while higher quartiles of diabetics were more treated with insulin. There were no differences in other previous medications (Tables 1 and 2).
Patients in lower quartiles of glycemia variation in both the nondiabetic (92.5%, 93.2%, 86.9%, 77.8%; P<.001) and diabetic group (81.9%, 83.3%, 71.9%, 64.6%; P<.001) had more frequent Killip class I on admission.
Regarding admission ECG, patients in higher glycemia variation groups had more frequent ST elevation (17.3%, 20.6%, 32.2%, 34.0%; P<.001) and atrial fibrillation (2.6%, 4.4%, 6.3%, 13.1%; P<.001) in nondiabetics, while there were no differences in occurrence of ST elevation (11.0%, 26.5%, 26.3%, 21.5%; P=.11) and atrial fibrillation (11.0%, 6.1%, 12.6%, 12.9%; P=.38) in diabetic groups.
Mean glycemia level at admission was 131.6 (48.3) mg/dL and 201 (81.6) mg/dL and fasting glycemia 120 (38.7) mg/dL and 177.6 (72) mg/dL in nondiabetics and diabetics, respectively. Magnitude of glycemia variation was correlated with higher levels of glycemia at admission and fasting glycemia (Tables 3 and 4).
Higher magnitude of glycemia variation was strongly correlated with higher necrosis and inflammation biomarkers in both groups, but was only associated with lower creatinine clearance in non-diabetics (Tables 3 and 4).
Regarding in-hospital drug therapy, nondiabetics in Q4 received more glycoprotein IIb/ IIIa inhibitors, diuretics and catecholamines and less beta-blockers, while diabetics received more diuretics and less ACE inhibitors and beta-blockers (Tables 5 and 6). There were no significant differences regarding other pharmacological therapies, reperfusion strategies and coronary anatomy, except less normal coronaries in higher quartiles of nondiabetics (Tables 5 and 6).
Left ventricular ejection fraction, evaluated by echocardiography, was worse in nondiabetics (58% [50%-60%], 55% [47.5%-60%], 52% [44%-58%], 50% [40%-58%]; P<.001) and diabetics (55.5% [46.3%-60%], 50% [43%-59%], 52.0% [45%-58%], 49% [38%-56%]; P=.016) with higher glycemia variations. These patients also had longer hospitalizations (nondiabetics, 4 [3-6], 5 [4-6], 5 [4-6], 5 [4-7] days, [P<.001]; and diabetics, 4 [3-5], 4 [3-6], 6 [4-7], 6 [4-8] days, [P<.001]).
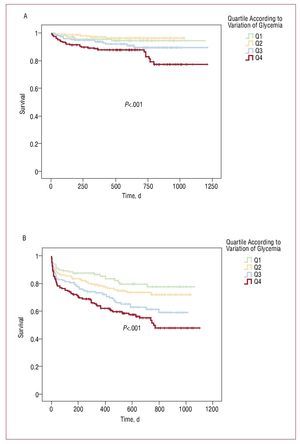
In diabetic patients there was no relationship between amplitude of glycemia variation and in-hospital mortality (3.3%, 7.3%, 7.5%, 7.5%; P=.27) and morbidity (5.3%, 7.1%, 6.2%, 8.2%; P=.50), and post-discharge endpoints (38%, 50%, 40.7%, 48.6%; P=.39) and mortality (13.2%, 17.1%, 14.5%, 20.0%; P=.37). In nondiabetics, no significant differences were observed regarding inhospital mortality (2.6%, 3.4%, 2.9%, 5.9%; P=.11) and morbidity (3.9%, 3.4%, 2.9%, 2.9%; P=.52); however, higher amplitude of glycemia variation was associated with higher post-discharge endpoints (22.7%, 26.4%, 35.7%, 44%; P<.001) and mortality (4.4%, 4.6%, 7.9%, 15.4%; P<.001) during follow-up. Figure 1 shows the Kaplan-Meier curves for post-discharge endpoints and follow-up mortality in the nondiabetic group.
Figure. Kaplan-Meyer curves for the follow-up mortality (A) and post-discharge endpoints (B) in nondiabetic population.
In the overall population, total number of inhospital morbidity events was 57, total number of in-hospital deaths was 52 and total number of post-discharge endpoints was 417 (of whom 122 were deaths).
Multivariate logistic regression analysis showed that higher magnitude of glycemia variation (Q4 vs Q1) was an independent predictor of post-discharge endpoints in nondiabetics, as were age and previous treatment with antiplatelet agents and ACE-inhibitors (Table 7).
DISCUSSION
In this manuscript we present data on a new parameter to evaluate metabolic control in ACS patients: the magnitude of glycemia variation. Our results show that magnitude of glycemia variation was rather useful in determining post-discharge outcome in the nondiabetic population, were it was an independent predictor of post-discharge endpoints in the mean 18-month follow-up. Logistic regression was also performed using post-ACS mortality (data not shown), but the magnitude of glycemia variation was not found to be an independent predictor.
According to the literature, hyperglycemia on hospital admission after an ACS is a common finding and should be considered an important marker of poor clinical outcome and increased mortality in patients with and without a history of diabetes.1-11
Although most studies have focused their attention on the prognostic value of blood glucose at admission, it represents only a single measurement in time and does not reflect the overall exposure to hyperglycemia during hospitalization.
There are several candidate measures to access the metabolic control in hospitalized patients. In our population, magnitude of glycemia variation during hospitalization was an independent predictor of post-discharge endpoints after ACS in nondiabetic patients.
Suleiman et al14 showed a graded relationship between both elevated fasting glycemia and admission glycemia and 30-day mortality in nondiabetic patients with AMI, suggesting that fasting glycemia is a more important predictor of 30-day mortality than admission glucose alone. Patients with both elevated admission glucose and elevated fasting glucose the next day had a 3-fold increase in mortality.
Similarly, failure of an elevated glucose level to normalize within 24 h of admission is associated with worse prognosis.15,16 A recently published study16 demonstrated that elevated admission and mean hospitalization glucose levels may be used to trigger a decision to institute intensive glucose control in hyperglycemic patients with AMI. Persistent hyperglycemia is a good discriminator of mortality, probably better than admission glucose alone in patients hospitalized with AMI.14-16
In our nondiabetic population, we found an important association between magnitude of glycemia variation and both post-discharge endpoints and mortality, unlike with in-hospital prognosis. In contrast, magnitude of glycemia variation was not related to worse prognosis in diabetic patients.
Our findings are in line with previous studies,2,8-10 which showed a different prognosis impact of stress hyperglycemia in patients without previously known diabetes and in diabetics.
There could be several explanations for this evidence. First, some patients without a history of diabetes who develop hyperglycemia in stressful situations are true diabetics or have impaired glucose tolerance. It has been reported that abnormal glucose tolerance is common among patients with AMI who have no previous diagnosis of diabetes17-19 and is a strong risk factor for future cardiovascular events.19
However, a recent study20 showed that two-thirds of patients with AMI who had no previous diagnosis of diabetes had abnormal glucose tolerance by OGTT one week after AMI, regardless of admission glucose levels, and admission hyperglycemia in nondiabetics did not represent previously undiagnosed abnormal glucose tolerance.
Second, hyperglycemia in patients without diabetes is more often a marker of stress response due to more extensive myocardial damage, as a greater degree of stress is necessary to achieve the hyperglycemic state because their metabolic control is usually normal.
Elevated glycemia after ACS in diabetic patients may be a surrogate for poor glycemic control, associated in our study with high short- and long-term mortality, although there was not a significant relationship between quartiles of admission glycemia and higher mortality.
However, observational studies cannot distinguish whether glucose levels are merely risk markers or direct mediators of outcome following AMI. Current evidence suggests that hyperglycemia is a mediator of worse prognosis, directly exacerbating myocardial damage. Experimental and clinical studies have shown that hyperglycemia per se exacerbates myocardial necrosis in AMI.21-24 Higher glucose levels in AMI patients have been associated with higher free fatty acid concentrations, insulin resistance and impaired myocardial glucose use, thus increasing oxygen consumption and potentially worsening ischemia.
Acute hyperglycemia is associated with numerous adverse effects which lead to a poor outcome in ACS patients: endothelial dysfunction, platelet hyperreactivity, increased cytokine activation, increased lipolysis and free fatty acid levels, decreased glycolysis and glucose oxidation, increased oxidative stress (increased myocardial apoptosis), impaired microcirculatory function ("no-reflow" phenomenon), impaired ischemic preconditioning, impaired insulin secretion and insulin-stimulated glucose uptake.24
Increased oxidative stress interferes with nitric oxide-mediated vasodilatation and reduces coronary blood flow at the microvascular level. In STEMI subjects, acute hyperglycemia is associated with decreased TIMI 3 flow before intervention, compared with euglycemia, and is the most important predictor of absence of coronary perfusion.25 It is interesting to note that in our study the performance of reperfusion therapy in STEMI patients did not independently influence the results (data not shown).
Acute hyperglycemia is associated with impaired microcirculatory function ("no reflow" on myocardial contrast echocardiography after percutaneous coronary intervention), even in the context of angiographically successful recanalization.4 Preexisting HbA1c levels and diabetes status do not differ between subsets with and without "no-reflow," suggesting that acute, and not chronic, hyperglycemia is the dominant factor.
There is increasing evidence that tight glycemic control for patients admitted to hospital improves clinical outcomes, especially for patients with cardiovascular disease.26,27 Recently, the DIGAMI 2 study27 strongly supported the concept, defended previously by van den Bergh,26 that a meticulous glucose control is an important goal to improve outcomes after ACS.
Compared to the other parameters of glucose metabolism control already available, namely admission glycemia and short-term glycemia normalization, the novel marker introduced in this study, magnitude of glycemia variation, has the advantage of being a more dynamic and "longitudinal" parameter, providing information about the extension of glycemia excursions during the entire ACS hospitalization, rather than only a single snapshot of glucose metabolism (like admission glycemia) or a series of snapshots regarding the first 24 h (like the glycemia normalization). Although more complex to measure, this may be a better way of assessing metabolic stress and metabolic control during ACS in nondiabetic patients. Further studies are warranted to determine if the magnitude of glycemia variation adds something to the predictive value provided by admission glycemia.
All of these data, together with the main findings of our study, clearly stress the importance of optimum metabolic control to prevent coronary events and their frequent poor outcome, especially in high-risk populations.
Study Limitations
This study was based on a database of 1210 consecutive patients admitted for ACS in a single centre. However, during this period some patients were admitted to the emergency room of our hospital with a possible ACS and died before being admitted in the coronary care unit (mainly just before or during the primary percutaneous coronary intervention attempt), which may have somehow influenced our results. Only 33 patients (2.7% of the population) were lost to follow-up, a figure within expected results for a single-centre registry. The assessment of post-discharge outcomes was made by review of medical records and telephone interview; therefore, some events may not have been adequately censored, especially if they occurred out of hospital or in other hospitals without patient referral to our centre. Causes of death were based on death certificates, if available, but in some cases the source of information was a patient's relative (therefore not always fully reliable). Although this was a single-centre study, we think its results can be extrapolated for other ACS populations because our demographic and clinical data are in line with those reported in most ACS registries published. However, large-scale clinical trials and registries are warranted to fully assess the predictive value of this and other metabolic parameters in ACS patients.
CONCLUSION
In this ACS population, magnitude of glycemia variation during hospitalization was an independent predictor of post-discharge endpoints in nondiabetic patients. This parameter, never before described, may be an important marker of metabolic control with prognostic value in these patients. This finding highlights an important potential opportunity to improve care and outcomes for hyperglycemic AMI patients without known diabetes.
ACKNOWLEDGMENTS
The statistical analysis was performed by Dr Adriana Belo from the National Centre for Data Collection in Cardiology, an entity of the Portuguese Society of Cardiology.
ABBREVIATIONS
ACS: acute coronary syndrome
AMI: acute myocardial infarction
NSTEMI: non-ST elevation myocardial infarction
STEMI: ST elevation myocardial infarction
SEE ARTICLE ON PAGES 1092-4
Correspondence: Dra. S. Reis Monteiro.
Departamento de Cardiologia. Hospital Universitario de Coimbra.
P. Prof. Mota Pinto. 3000-075 Coimbra. Portugal.
E-mail: silvia.reis.monteiro@gmail.com
Received February 9, 2009.
Accepted for publication April 15, 2009.