Sustained monomorphic ventricular tachycardia (SMVT) is a major cause of morbidity and sudden cardiac death in patients with structural heart disease, mainly in the context of ischemic heart disease.1 The treatment of SMVT and prevention of sudden death in this group of patients is sustained by 2 main treatments: the implantable cardioverter defibrillator (ICD) and radiofrequency ablation, whose future development will be based on a more complete knowledge of the pathophysiological substrate of SMVT.
THERAPEUTIC OPTIONS FOR PATIENTS WITH POSTINFARCTION VENTRICULAR TACHYCARDIASImplantable Cardioverter DefibrillatorThe ICD has been shown to reduce overall mortality and the incidence of arrhythmia compared with drug therapy in both secondary and primary prevention.2,3 However, it is not an innocuous therapy since the shocks are painful, give rise to an important psychological impact that significantly impairs patients’ quality of life, shorten the useful life of the devices, and increase the mortality rate.4 To a certain extent, the problem is ameliorated by antitachycardia pacing, but this strategy does not prevent it completely, since the patients who undergo it are at risk for new episodes that do not respond to this therapy and, therefore, require shocks.5 Moreover, the ICD does not eliminate the arrhythmogenic substrate and, thus, does not prevent the development of ventricular tachyarrhythmias.6
Radiofrequency AblationNonrandomized studies carried out in the last decade began to indicate that radiofrequency ablation might be effective for the treatment of SMVT, as it reduced recurrences by 38% compared with drug therapy, although the complications rate was high.7 However, the first randomized clinical trials were not carried out until relatively recently. The SMASH-VT trial8 compared ablation (substrate-based) and standard medical treatment in patients with hemodynamically unstable ventricular tachycardia (VT), patients with syncope and inducible VT, and patients who had undergone implantation of an ICD for primary prevention and had received an appropriate shock. In the group of patients who underwent ablation, ICD interventions were reduced by 73% and there was a nonsignificant trend toward a reduction in mortality. The VTACH trial9 studied the “prophylactic” effect of ablation in 107 patients with chronic ischemic heart disease who were scheduled to undergo ICD implantation following an episode of hemodynamically tolerated SMVT. After 2 years of follow-up, 47% of the patients who had undergone ablation were free of recurrences, compared with 29% in the group without ablation, and the difference was especially marked in patients with a left ventricular ejection fraction > 30%. Thus, these 2 reports demonstrate the efficacy of ablation in 2 different but complementary scenarios; on the one hand, patients with poorly tolerated VT or VT with syncope and, on the other, those who tolerated VT, although their ventricular function had deteriorated little. However, there is no clinical evidence showing that ablation reduces mortality, except in patients with electrical storm.10
These data justify performing ablation as an adjuvant treatment in patients with ICD from the onset of SMVT, rather than waiting until the patient has experienced several shocks to resort to this approach.
SUBSTRATE ABLATIONThe objective of conventional mapping is to define the critical isthmus of slow conduction in the VT circuit for subsequent ablation using pace, activation and entrainment mapping. Because these maneuvers should be carried out during tachycardia, their major limitations are the need for multiple inductions in some patients, noninducibility in others, and the poor hemodynamic tolerance of up to 50% of the induced VT.11 Taken together, these circumstances result in very long procedures requiring repeated cardioversion of the patients, with the potential complications mentioned above.4
To resolve these problems, techniques for ablation of the arrhythmic substrate have emerged for the purpose of defining, with the patient in sinus rhythm, the scar tissue related to the tachycardias.
Pathophysiological Substrate: the ScarClassical studies performed in the 1980s demonstrated that most postinfarction SMVT are maintained by a reentry mechanism located in the scar that develops after acute myocardial infarction.12,13 The main histological characteristic of this scar with respect to the reentry mechanism is the presence of heterogeneous tissue (HT) in its interior, that is, bundles of viable myocytes surrounded by connective tissue with reduced conduction velocity.14 These clusters of myocytes form channels of slow conduction (CSC) that connect the interior of the scar with areas of healthy tissue and constitute the critical isthmuses of the SMVT circuits, whereas the areas of dense scar create regions of anatomical block around the CSC.13 These structures promote the development of unidirectional block and reentry.
The association of CSC and HT with SMVT is reflected in a number of reports. The extent of the HT has been related to the inducibility of VT, to the cycle length,15 and to mortality.16,17 Likewise, channels of HT have been observed more frequently in patients with clinical VT than in those with no clinical history of VT.18 That is, HT and the presence of CSC in the scar are the main determinants of the development of SMVT in patients with chronic myocardial infarction.
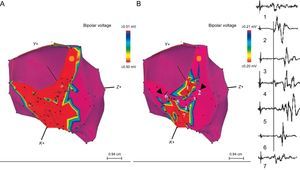
Substrate Ablation TechniqueAs mentioned above, the areas of slow conduction of SMVT are related to the presence within the scars of viable myocytes surrounded by fibrous tissue, and they have few connections, which slows conduction. Electrograms corresponding to these areas have low voltage and multiple components, some of which are isolated and late; these electrograms show isolated components or late potentials (EIC/LP). The myocytes cluster to form CSC that are identified within the scar (voltage < 1.50mV) and, specifically, within the dense scar (voltage < 0.50mV). These CSC are identified by adjusting the voltage range, and it is important to sequentially lower the range to 0.10mV (Figure 1).19 However, the mere existence of a “voltage channel” is insufficient to define the existence of a CSC. It is necessary to create a detailed map of the EIC/LP and demonstrate their presence in the channels by means of a logical sequence, since their importance in the identification of slow conduction isthmuses has been established.20,21 The map can be generated with the patient in sinus rhythm or during right ventricular apex pacing, an approach that facilitates the identification of these EIC/LP which, since they are activated from several sources, could otherwise remain undetected.20
Effect of voltage adjustment on the definition of the channels of slow conduction within the scar. A: this inferior view shows the dense scar in red when the voltage cutoff was 0.50mV. B: when the voltage cutoff was lowered to 0.20mV, the presence of a channel that runs through the dense scar from the septal to the lateral region was observed. To the right of the figure: the sequence of electrograms obtained in the channel during right ventricular apical pacing. Reproduced with permission of Arenal et al.19
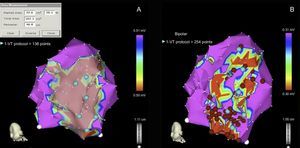
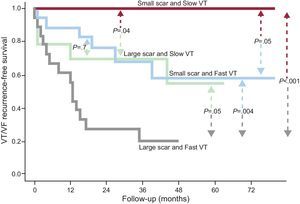
The ablation of CSC and EIC/LP related to clinical or induced VT has been shown to significantly reduce the rate of recurrence of ventricular arrhythmias and to lower the risk of receiving ICD shocks.8,19,22 On the basis of these data, to simplify the procedure and treat patients showing no electrocardiographic evidence of spontaneous VT, techniques have emerged to achieve the complete elimination of the endocardial substrate for SMVT. This consists of homogenization of the entire scar by means of ablation of all the CSC and EIC/LP (Figure 2), whether or not a relationship with clinical VT has been demonstrated. When this approach was employed in a group of 59 patients, the recurrence-free rate was 81% after a mean follow-up of 40 months. The VT cycle length and size of the scar were independent predictors related to the absence of recurrences (Figure 3), while the noninducibility of clinical VT had no effect on efficacy.23 This strategy for the homogenization of the scar tissue has been employed by other groups and has even been extended to epicardium.22,24
Procedure for the complete ablation of the arrhythmic substrate. A: identification of dense scar, defined by a voltage < 0.50mV. B: complete substrate ablation following identification of the electrograms with isolated components and/or late potentials and the channels of slow conduction found on adjustment of the voltage cutoff. VF, ventricular fibrillation; VT, ventricular tachycardia. Reproduced with permission of Arenal et al.23
Recurrence-free survival curves according to scar size and ventricular tachycardia cycle length. Dense scar and clinical ventricular tachycardia cycle length are presented as dichotomous variables, using the median value as the cutoff point: large or small scar size (> 25cm2 or ≤ 25cm2) according to the electroanatomic map and slow or fast clinical ventricular tachycardia (≤ 350ms or > 350ms). VF, ventricular fibrillation; VT, ventricular tachycardia. Reproduced with permission of Arenal et al.23
Two recent studies report that the isthmuses of some VT are not related to the CSC or to EIC/LP.23,25 In the first study, the VT unrelated to CSC were some of the very rapid tachycardias (cycle length < 300ms) that were related to electrograms with multiple components located on the scar borders.23In the second, the VT unrelated to CSC were significantly slower than those that were related (440 [40] ms vs 377 [67] ms; P =.016). This study, however, has several limitations: in addition to its retrospective design, complete mapping was not carried out during tachycardia and the circuit exit site was not located; thus, the possibility that it was in some channel near the point of the recording of the middiastolic electrograms cannot be ruled out. Even so, when we are dealing with very rapid or very slow SMVT, we should take these limitations into account and perform more detailed mapping both in sinus rhythm and during tachycardia.
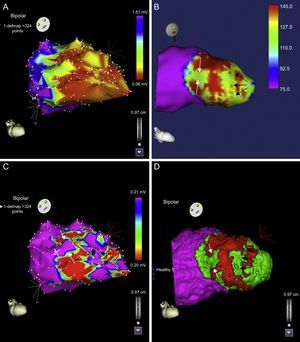
Noninvasive Substrate Identification: Role of Magnetic Resonance ImagingMagnetic resonance provides high-definition images of cardiac anatomy, and late gadolinium enhancement allows visualization of the regions of scarring and HT. This technique shows a good correlation with endocardial voltage mapping with regard to both the localization and extent of the scar and the arrhythmic substrate, since the critical sites of the circuits are located in the zones of HT.26 In fact, the extent of this HT has been correlated with the inducibility of VT16 and with mortality,17 although its specific role in arrhythmic risk stratification has yet to be defined. In an attempt to characterize the arrhythmic substrate within the scar in greater detail, our group has succeeded in acquiring what we refer to as signal intensity maps, obtained by means of the postprocessing of late-enhancement magnetic resonance images, to locate HT within the scar and thus define CSC (Figure 4). These maps show a good correlation in the characterization of the scar and localization of the channels with respect to conventional endocardial voltage mapping.27
Relationship between endocardial voltage mapping and signal intensity mapping. A: endocardial voltage map showing anterior myocardial infarction. B: subendocardial signal intensity map, which agrees with the voltage map and shows a channel parallel to the mitral annulus (black arrows) and another incomplete channel perpendicular to the previous channel (white arrows). C: voltage map with cutoff at 0.20mV showing a better definition of the channels than in A. D: integration of the 2 maps. Reproduced with permission of Pérez-David et al.27
Future advances in imaging techniques could help in the noninvasive characterization of the substrate, contributing to an optimal definition of the scar and eliminating the errors that are sometimes produced due to the poor contact of the catheter or the interposition of structures such as epicardial fat. The integration of the information provided by magnetic resonance into navigator-gated mapping could result in more accurate maps, shorten procedure times, and improve the long-term outcome of ablation.
THE IDEAL ABLATION PROCEDURE FOR SCAR-RELATED VENTRICULAR TACHYCARDIASIn our opinion, based on previously presented data, we should forget the differences between conventional and substrate ablation, and the very terms themselves, and design a standard noninvasive procedure based on detailed 3-dimensional characterization of the scar, in which the spread to epicardium would also be assessed, and on the performance of 3-dimensional mapping to identify areas of slow conduction and establish their relationship to the clinical tachycardias, to proceed to their complete elimination. Induction of tachycardia and mapping during its presence should be the final step of the procedure, and every attempt should be made to prevent the occurrence of shocks during the intervention because of their deleterious effect.
Thus, the steps to be followed in any ablation procedure would be the following:
- 1.
Noninvasive reconstruction and characterization of the scar (magnetic resonance).
- 2.
Three-dimensional mapping of the scar with the support of noninvasive imaging and tagging of the CSC and EIC/LP.
- 3.
Identification of clinical tachycardias by means of pace mapping.
- 4.
Ablation of all these substrate tags and documented areas of slow conduction.
- 5.
Assessment of the inducibility and, in the case of persistence, repetition of endocardial mapping techniques.
- 6.
Examination of the epicardium if inducibility persists or if no areas of endocardial substrate are found.
Based on the existing data, ablation should be routinely performed in the treatment of patients with ischemic heart disease who are admitted to hospital with SMVT or ICD shocks to prevent their recurrence, but this technique should not be employed alone and should be accompanied by ICD implantation. In this setting, strategies based on complete substrate ablation offer notable advantages over conventional ablation techniques.
The use of magnetic resonance could be highly useful both in the evaluation of arrhythmic risk and in the planning and performance of the electrophysiological study. Thus, its implementation should be considered prior to ICD implantation. Nevertheless, further studies will be needed to confirm its true clinical impact.
FUNDINGThis report has been financed by RECAVA (Cooperative Cardiovascular Disease Research Network [Red Temática de Investigación en Enfermedades Cardiovasculares]), Instituto de Salud Carlos III, Spanish Ministry of Health, and by the Foundation for Biomedical Research of Hospital General Universitario Gregorio Marañón, Spain.
CONFLICTS OF INTERESTNone declared.