There is a discrepancy between risk assessment based on cardiovascular risk factors (CVRF) and atheromatosis burden. The objective was to identify the prevalence of subclinical diseases with common risk factors, such as atheromatosis, occult kidney disease, prediabetes, and diabetes in a middle-aged population with low-to-moderate cardiovascular risk; to assess the vascular distribution, and severity of subclinical atheromatosis.
MethodsRandomized, interventional, longitudinal clinical trial. The intervention consisted of vascular ultrasound examination in the carotid and femoral arteries assessing 12 territories, combined with clinical, anthropometric, lifestyle, and biochemical parameters. Inclusion criteria consisted of women (aged 50-70 years) and men (aged 45-65 years) with at least 1 CVRF. Exclusion criteria consisted of a clinical history of diabetes, chronic kidney disease, or a prior CV event. Here, baseline characteristics of the ILERVAS cohort are shown.
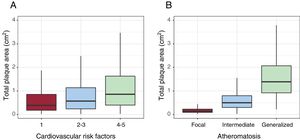
ResultsA total of 8330 middle-aged asymptomatic participants, 50.7% women, were enrolled. The presence of 1-2 CVRF was found in 74.8% and adherence to the Mediterranean diet was low in 52.8%. Several previously unknown chronic diseases were diagnosed, such as dyslipidemia (21.1%), hypertension (15.3%), kidney disease (15.4%), obesity (10.6%), and diabetes (2.3%). Subclinical atheromatosis was found in 71.4% of participants, localized in common femoral (54.5%), carotid bifurcation (41.1%) and internal carotid (22%). Intermediate atheromatosis (2-3 territories with atheroma plaque) was found in 32.6%, and generalized atheromatosis (>3 territories) in 19.7. Total plaque area was higher in men (0.97 cm2 vs 0.58 cm2, P<.001). Total plaque area was also higher in the femoral artery, and increased with the number of CVRF.
ConclusionsSubclinical atheromatosis was highly prevalent in a middle-aged population with low-to moderate cardiovascular risk, with 1 in 5 participants having generalized atheromatosis.
ClinicalTrials.gov Identifier: NCT03228459
Keywords
Despite the identification of traditional cardiovascular risk factors (CVRF) in the early 1960s, and many nontraditional factors in the following decades, atheromatous cardiovascular disease (ACVD) is the leading cause of mortality and disability in most countries, causing one third of deaths.1 Atheromatosis develops insidiously and is usually widespread by the time of symptom's occurrence. Thus, early detection of subclinical atheromatosis is of paramount importance. The most widely used system for cardiovascular risk assessment is the Framingham risk score.2 Nevertheless, there are significant differences with the European population that preclude its direct application to our population.3 Therefore, the use of the European Systematic Coronary Risk Evaluation (SCORE)4 and the Framingham-REGICOR (Registre Gironí del Cor) risk charts are recommended in Spain for stratifying cardiovascular disease risk.5 Based on the 2019 clinical guidelines, patients are classified according to their SCORE as very high risk (SCORE ≥ 10% for 10-year risk of fatal CVD), high risk (≥ 5% and <10%), moderate risk (≥ 1% and <5%), and low risk (< 1%). This stratification has clinical implications. Thus, the treatment goal for low-density lipoprotein cholesterol (LDL-C) in patients at high risk is 70mg/dL (1.8 mmol/L) and 55mg/dL (1.4 mmol/L) in patients at very high risk.4
Multiple clinical studies have analyzed atheromatosis in high-risk cardiovascular populations such as persons with diabetes,6 familiar hypercholesterolemia,7 and chronic kidney disease.8 However, there is a high prevalence of atheromatosis among individuals in the general population.9,10 Indeed, there is a mismatch between the number of traditional risk factors and cardiovascular events, and atheromatosis-related adverse events are frequent even in low-to-moderate cardiovascular risk individuals.11 Therefore, better risk stratification methods are urgently needed.
The association of atheroma plaque with cardiovascular events has been extensively confirmed.12 In addition, multiterritorial evaluation increases its predictive value in cardiovascular mortality.13
Therefore, new longitudinal population-based studies focused on subclinical atheromatosis addressing its prevalence, multiterritorial extent, and progression in low-to-moderate cardiovascular risk individuals could change the paradigm, namely to treat early subclinical disease instead of cardiovascular risk factors.
Based on the aforementioned data, the ILERVAS study was designed as a randomized, interventional, longitudinal clinical trial. The primary objectives were: a) to assess the prevalence, vascular distribution, severity, and progression of subclinical atheromatosis in a middle-aged population with low-to-moderate cardiovascular risk; b) to reveal potential predictor factors of generalized atheromatosis beyond traditional cardiovascular risk factors; c) to identify the prevalence of subclinical diseases with common risk factors such as occult chronic kidney disease, prediabetes, diabetes, respiratory diseases, and metabolic syndrome; and d) to assess the impact of subclinical atheromatosis detection on the incidence of cardiovascular events during a 10-year follow-up period. Here, baseline characteristics of the ILERVAS cohort focused on atheromatosis are shown. Subclinical atheromatosis assessed in 12 territories and plaque area were combined with clinical, anthropometric, lifestyle, and biochemical parameters.
METHODSThe ILERVAS study is an ongoing randomized, interventional, longitudinal clinical trial with 2 arms: a) intervention group, hereafter called the mobile unit follow-up group, and b) no intervention group, hereafter called the electronic medical record follow-up group, in a low-to-moderate cardiovascular risk population of the province of Lleida, Spain (ClinicalTrials.gov identifier: NCT03228459).
In the mobile unit follow-up group, participants were enrolled between January 2015 and December 2018 from 32 primary basic health areas of the province of Lleida. The inclusion criteria were as follows: women aged 50 to 70 years and men aged 45 to 65 years with at least 1 of the following cardiovascular disease risk factors: hypertension, dyslipidemia, obesity (defined by body mass index [BMI] ≥ 30), smoking, and/or a first-degree relative who developed premature cardiovascular disease (with a threshold at age 55 years for men and 65 years for women). The exclusion criteria consisted of a clinical history of diabetes, chronic kidney disease, cardiovascular disease (angina, myocardial infarction, stroke, peripheral arterial disease, intestinal, or other ischemia), history of arterial surgery, active neoplasia, life expectancy less than 18 months, long-term home care, and/or institutionalized population. Participants will be reevaluated in the mobile unit after 4 years. A follow-up period of 10 years was established to monitor cardiovascular and noncardiovascular morbidity and mortality.
The study population was allocated to the groups by stratified sampling from the primary care electronic clinical history database of the Catalan Health Service (see supplementary data for further details). All patients provided signed informed consent. The protocol was approved by the Ethics Committee of The Catalan Health Service (Ref. CEIC-1410 Hospital Arnau de Vilanova, Lleida, Spain). The study was conducted according to the principles of the Declaration of Helsinki. A detailed description of methods is included in the supplementary data.
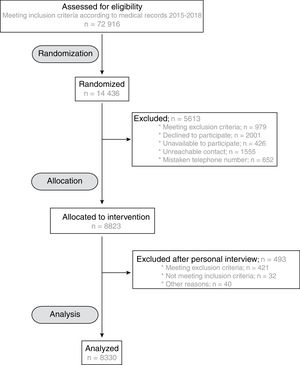
RESULTSBaseline characteristics of the ILERVAS cohortThe ILERVAS study recruited a total of 8330 middle-aged participants with no prior cardiovascular events from January 2015 to December 2018. Figure 1 shows the flow chart of participant selection from the candidate population.
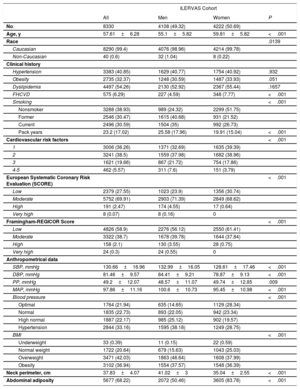
The patients’ baseline clinical characteristics are shown in table 1. The average age was 55.1 years for men (between 45 and 65 years) and 59.8 years for women (between 50 and 70 years). Most (99.4%) of them were Caucasian. Dyslipidemia was the most frequent registered cardiovascular risk factor (54.3% of the study population), followed by hypertension (40.9%) and obesity (32.4%). None of them showed differences by sex. Active smokers accounted for 35% of men (with an average of 25.6 pack-years of tobacco) and 26.7% of women (with an average of 19.9 pack-years of tobacco) (P <.001). The presence of 1 or 2 cardiovascular risk inclusion criteria was present in 70.7% of men compared with 78.4% of women. Older participants had a higher number of risk factors. The prevalence of 1 CVRF was 45.3% in men aged 45-49 years and was 25.8% in men aged 60 to 65 years. A similar tendency was observed in both sexes (figure 1 of the supplementary data). Most (97.5%) participants had low-to-moderate risk determined by the European Systematic Coronary Risk Evaluation (SCORE) score. Similarly, the Framingham-REGICOR score revealed that 97.6% of participants had low-to-moderate risk (table 1).
Patients’ baseline clinical characteristics
| ILERVAS Cohort | ||||
|---|---|---|---|---|
| All | Men | Women | P | |
| No. | 8330 | 4108 (49.32) | 4222 (50.69) | |
| Age, y | 57.61±6.28 | 55.1±5.82 | 59.81±5.82 | <.001 |
| Race | .0139 | |||
| Caucasian | 8290 (99.4) | 4076 (98.96) | 4214 (99.78) | |
| Non-Caucasian | 40 (0.6) | 32 (1.04) | 8 (0.22) | |
| Clinical history | ||||
| Hypertension | 3383 (40.85) | 1629 (40.77) | 1754 (40.92) | .932 |
| Obesity | 2735 (32.37) | 1248 (30.59) | 1487 (33.93) | .051 |
| Dyslipidemia | 4497 (54.26) | 2130 (52.92) | 2367 (55.44) | .1657 |
| FHCVD | 575 (6.29) | 227 (4.59) | 348 (7.77) | <.001 |
| Smoking | <.001 | |||
| Nonsmoker | 3288 (38.93) | 989 (24.32) | 2299 (51.75) | |
| Former | 2546 (30.47) | 1615 (40.68) | 931 (21.52) | |
| Current | 2496 (30.59) | 1504 (35) | 992 (26.73) | |
| Pack years | 23.2 (17.02) | 25.58 (17.96) | 19.91 (15.04) | <.001 |
| Cardiovascular risk factors | <.001 | |||
| 1 | 3006 (36.26) | 1371 (32.69) | 1635 (39.39) | |
| 2 | 3241 (38.5) | 1559 (37.98) | 1682 (38.96) | |
| 3 | 1621 (19.66) | 867 (21.72) | 754 (17.86) | |
| 4-5 | 462 (5.57) | 311 (7.6) | 151 (3.79) | |
| European Systematic Coronary Risk Evaluation (SCORE) | <.001 | |||
| Low | 2379 (27.55) | 1023 (23.9) | 1356 (30.74) | |
| Moderate | 5752 (69.91) | 2903 (71.39) | 2849 (68.62) | |
| High | 191 (2.47) | 174 (4.55) | 17 (0.64) | |
| Very high | 8 (0.07) | 8 (0.16) | 0 | |
| Framingham-REGICOR Score | <.001 | |||
| Low | 4826 (58.9) | 2276 (56.12) | 2550 (61.41) | |
| Moderate | 3322 (38.7) | 1678 (39.78) | 1644 (37.84) | |
| High | 158 (2.1) | 130 (3.55) | 28 (0.75) | |
| Very high | 24 (0.3) | 24 (0.55) | 0 | |
| Anthropometrical data | ||||
| SBP, mmHg | 130.66±16.96 | 132.99±16.05 | 128.61±17.46 | <.001 |
| DBP, mmHg | 81.46±9.57 | 84.41±9.21 | 78.87±9.13 | <.001 |
| PP, mmHg | 49.2±12.07 | 48.57±11.07 | 49.74±12.85 | .009 |
| MAP, mmHg | 97.86±11.16 | 100.6±10.73 | 95.45±10.98 | <.001 |
| Blood pressure | <.001 | |||
| Optimal | 1764 (21.94) | 635 (14.65) | 1129 (28.34) | |
| Normal | 1835 (22.73) | 893 (22.05) | 942 (23.34) | |
| High normal | 1887 (22.17) | 985 (25.12) | 902 (19.57) | |
| Hypertension | 2844 (33.16) | 1595 (38.18) | 1249 (28.75) | |
| BMI | <.001 | |||
| Underweight | 33 (0.39) | 11 (0.15) | 22 (0.59) | |
| Normal weight | 1722 (20.64) | 679 (15.63) | 1043 (25.03) | |
| Overweight | 3471 (42.03) | 1863 (46.64) | 1608 (37.99) | |
| Obesity | 3102 (36.94) | 1554 (37.57) | 1548 (36.39) | |
| Neck perimeter, cm | 37.83±4.07 | 41.02±3 | 35.04±2.55 | <.001 |
| Abdominal adiposity | 5677 (68.22) | 2072 (50.46) | 3605 (83.78) | <.001 |
BMI, body mass index; DBP, diastolic blood pressure; FHCVD, family history of cardiovascular disease; MAP, mean arterial pressure; PP, pulse pressure; REGICOR, REGICOR, Registre Gironí del Cor; SBP, systolic blood pressure.
Values are shown as No. (%) for qualitative variables and as mean±standard deviation for normally distributed quantitative variables. Sample weights were applied in the analysis. Clinical history data were obtained from electronic medical records and refer to patients who had a prior clinical diagnosis of hypertension, obesity, dyslipidemia, a first-degree relative who developed premature CVD (with a threshold at age 55 years for men or 65 years for women), or smoking. Optimal blood pressure was defined as SBP <120mmHg and DBP <80; normal as SBP 120-129 and/or DBP 80-84; high normal as SBP 130-139 and/or DBP 85-89; hypertension as SBP≥140 and/or DBP≥90; Underweight was defined as a BMI <18.5kg/m2, normal weight as 18.5-24.9, overweight 25-29.9, and obesity≥30. Abdominal adiposity was defined as an abdominal perimeter≥88 cm in women or≥102 in men.
Women had lower blood pressure, body mass index, and neck perimeter values than men but much higher abdominal adiposity (83.8% of women vs 50.5% of men, P <.001).
Men took more vigorous and moderate physical activity than women. Men did 961.6minutes/wk of total activity, whereas women did 834.7 (P=.003). However, 60.95% of participants did low physical activity, 33.8% moderate, and 5.3% vigorous activity. A total of 52.8% of participants showed low adherence to Mediterranean diet, and 39.8% had very low adherence (table 1 of the supplementary data).
Normal respiratory function was observed in 68.5% of men and 76.1% of women. Respiratory pattern alterations were more prevalent in men. Almost half (45.2%) of participants who could be classified by the Berlin questionnaire showed a high risk of obstructive sleep apnoea, and 2.1% had excessive daytime sleepiness (table 2 of the supplementary data).
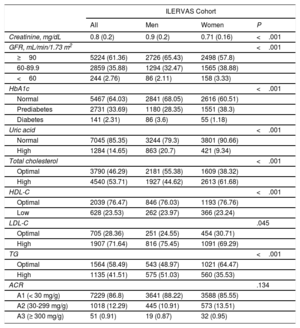
Baseline biochemical analysis revealed that 2.8% of participants had an estimated glomerular filtration rate <60mL/min/1.73 m2; 33.7% of prediabetes individuals (glycosylated haemoglobin, 5.7-6.49%), 2.3% of diabetes individuals (HbA1c ≥ 6.5%), and 14.7% with high uric acid (≥ 7.2mg/dL in men and ≥ 6.4 in women). High cholesterol levels (≥ 200mg/dL) were observed in 53.7% of participants, while 71.6% of participants showed high LDL-C (≥130 mg/dL), 41.5% high triglycerides (≥ 150mg/dL), and 23.5% low high-density lipoprotein cholesterol (≤ 40mg/dL in men and ≤ 50 in women). Urine analysis showed that 12.3% of participants had a 30-300mg/g albumin/creatinine ratio (table 2). The intraclass correlation coefficient between the point-of-care methods used in the ILERVAS mobile unit and gold standard biochemical methods are shown in table 3 of the supplementary data.
Baseline biochemical parameters
| ILERVAS Cohort | ||||
|---|---|---|---|---|
| All | Men | Women | P | |
| Creatinine, mg/dL | 0.8 (0.2) | 0.9 (0.2) | 0.71 (0.16) | <.001 |
| GFR, mL/min/1.73 m2 | <.001 | |||
| ≥90 | 5224 (61.36) | 2726 (65.43) | 2498 (57.8) | |
| 60-89.9 | 2859 (35.88) | 1294 (32.47) | 1565 (38.88) | |
| <60 | 244 (2.76) | 86 (2.11) | 158 (3.33) | |
| HbA1c | <.001 | |||
| Normal | 5467 (64.03) | 2841 (68.05) | 2616 (60.51) | |
| Prediabetes | 2731 (33.69) | 1180 (28.35) | 1551 (38.3) | |
| Diabetes | 141 (2.31) | 86 (3.6) | 55 (1.18) | |
| Uric acid | <.001 | |||
| Normal | 7045 (85.35) | 3244 (79.3) | 3801 (90.66) | |
| High | 1284 (14.65) | 863 (20.7) | 421 (9.34) | |
| Total cholesterol | <.001 | |||
| Optimal | 3790 (46.29) | 2181 (55.38) | 1609 (38.32) | |
| High | 4540 (53.71) | 1927 (44.62) | 2613 (61.68) | |
| HDL-C | <.001 | |||
| Optimal | 2039 (76.47) | 846 (76.03) | 1193 (76.76) | |
| Low | 628 (23.53) | 262 (23.97) | 366 (23.24) | |
| LDL-C | .045 | |||
| Optimal | 705 (28.36) | 251 (24.55) | 454 (30.71) | |
| High | 1907 (71.64) | 816 (75.45) | 1091 (69.29) | |
| TG | <.001 | |||
| Optimal | 1564 (58.49) | 543 (48.97) | 1021 (64.47) | |
| High | 1135 (41.51) | 575 (51.03) | 560 (35.53) | |
| ACR | .134 | |||
| A1 (< 30 mg/g) | 7229 (86.8) | 3641 (88.22) | 3588 (85.55) | |
| A2 (30-299 mg/g) | 1018 (12.29) | 445 (10.91) | 573 (13.51) | |
| A3 (≥ 300 mg/g) | 51 (0.91) | 19 (0.87) | 32 (0.95) | |
ACR, albumin/creatinine ratio; GFR, glomerular filtration rate; Hb1Ac, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
Values are shown as No. (%) for qualitative variables and as mean (standard deviation) for normally distributed quantitative variables. Sample weights were applied in the analysis. Normal HbA1c was defined as HbA1c ≤ 5.69, prediabetes as HbA1c 5.7-6.49, and diabetes as HbA1c ≥ 6.5. High uric acid was defined as ≥ 6.4mg/dL in women and ≥ 7.2 in men. High cholesterol was defined as ≥ 200mg/dL. Low HDL-C was defined as ≤ 50mg/dL in women and ≤ 40 in men. LDL-C was considered high at ≥ 130mg/dL. TG were considered high at ≥ 150mg/dL. LDL-C, HDL-C and TG were evaluated only in participants with total cholesterol ≥ 200mg/dL after 6hours of fasting or when total cholesterol was ≥ 250mg/dL regardless of fasting hours.
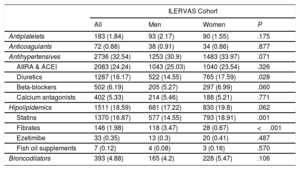
Drug treatments are listed in table 3. The most frequent was antihypertensive medication (32.5%), while 18.6% of participants were receiving treatment for hyperlipidemia (16.9% in statins, 1.98% fibrates, 0.4% ezetimibe, and 0.1% fish oil supplements).
Pharmacological treatments
| ILERVAS Cohort | ||||
|---|---|---|---|---|
| All | Men | Women | P | |
| Antiplatelets | 183 (1.84) | 93 (2.17) | 90 (1.55) | .175 |
| Anticoagulants | 72 (0.88) | 38 (0.91) | 34 (0.86) | .877 |
| Antihypertensives | 2736 (32.54) | 1253 (30.9) | 1483 (33.97) | .071 |
| AIIRA & ACEI | 2083 (24.24) | 1043 (25.03) | 1040 (23.54) | .326 |
| Diuretics | 1287 (16.17) | 522 (14.55) | 765 (17.59) | .028 |
| Beta-blockers | 502 (6.19) | 205 (5.27) | 297 (6.99) | .060 |
| Calcium antagonists | 402 (5.33) | 214 (5.46) | 188 (5.21) | .771 |
| Hipolipidemics | 1511 (18.59) | 681 (17.22) | 830 (19.8) | .062 |
| Statins | 1370 (16.87) | 577 (14.55) | 793 (18.91) | .001 |
| Fibrates | 146 (1.98) | 118 (3.47) | 28 (0.67) | <.001 |
| Ezetimibe | 33 (0.35) | 13 (0.3) | 20 (0.41) | .487 |
| Fish oil supplements | 7 (0.12) | 4 (0.08) | 3 (0.16) | .570 |
| Broncodilators | 393 (4.88) | 165 (4.2) | 228 (5.47) | .106 |
ACEI, angiotensin-converting enzyme inhibitors; AIIRA, angiotensin II receptor antagonists.
Values are shown No. (%). Sample weights were applied in the analysis.
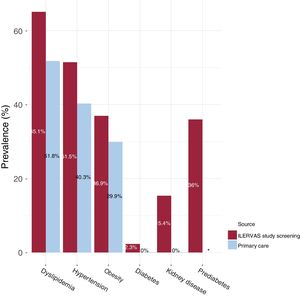
Data collected from the electronic medical record database of primary care were compared with data obtained in the ILERVAS mobile unit. The ILERVAS study detected 15.3% of undiagnosed hypertensive participants (95% confidence interval [95%CI], 14-16.6), 10.6% of undiagnosed obese [95%CI, 9.6-11.7], and 21.1% of undiagnosed dyslipidemic individuals [95%CI, 19.7-22.6%]. Additionally, a 15.4% of occult kidney disease [95%CI, 14.1-16.7], and 2.3% of undiagnosed diabetes [95%CI, 1.7-3.02] were detected (figure 2).
New clinical findings in the ILERVAS study. The prevalence of dyslipidemia, hypertension, obesity, diabetes, kidney disease, and prediabetes described in the electronic medical record database of primary care were compared with the findings in the ILERVAS mobile unit. *The prevalence of prediabetes was not obtained from the electronic medical record database of primary care.
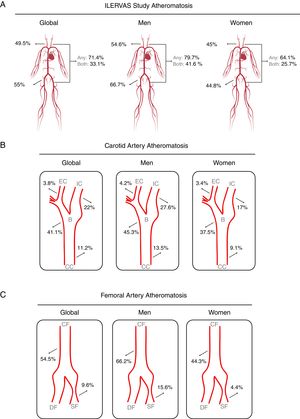
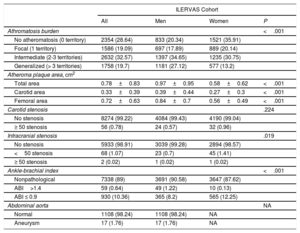
The presence of atheroma plaque was found in at least 1 explored territory in 71.4% of participants, and it was more prevalent in men (79.7% in men vs 64.1% in women; P <.001). Atheromatosis was more prevalent in femoral than in carotid arteries. Thus, 49.5% of participants showed atheroma plaques in carotid arteries and 55% in femoral arteries. Strikingly, 33.1% had plaques in carotid and femoral arteries, simultaneously. Similarly, men displayed atheroma plaque simultaneously in both vascular beds more frequently than women (men: 41.6% vs women: 25.7%; P <.001) (figure 3A).
Prevalence of subclinical atheromatosis by vascular territories in the ILERVAS study. Global (A), carotid artery (B), and femoral artery (C) territory distribution. B, carotid bifurcation; CC, carotid common; CF, common femoral; DF, deep femoral artery; EC, external carotid; IC, internal carotid; SF, superficial femoral artery.
Carotid atheromatosis was more prevalent in bifurcation (41.1%) followed by internal carotid (22%) and common carotid (11.2%). Femoral atheromatosis was more frequent in common femoral (54.5%) followed by superficial femoral (9.6%) (figure 3B,C).
No atheroma plaque was found in 28.6% of participants. Focal atheromatosis (defined by 1 territory with plaque out of 12) was found in 19.1%. Intermediate atheromatosis (defined by 2-3 territories with plaque out of 12) was found in 32.6%, and generalized atheromatosis (> 3 territories) was found in 19.7%. Men showed an increased burden of vascular disease (intermediate, 34.7% vs 30.8%; and generalized, 27.1% vs 13.2%; P <.001) (table 4).
Subclinical vascular assessment
| ILERVAS Cohort | ||||
|---|---|---|---|---|
| All | Men | Women | P | |
| Athromatosis burden | <.001 | |||
| No atheromatosis (0 territory) | 2354 (28.64) | 833 (20.34) | 1521 (35.91) | |
| Focal (1 territory) | 1586 (19.09) | 697 (17.89) | 889 (20.14) | |
| Intermediate (2-3 territories) | 2632 (32.57) | 1397 (34.65) | 1235 (30.75) | |
| Generalized (> 3 territories) | 1758 (19.7) | 1181 (27.12) | 577 (13.2) | |
| Atheroma plaque area, cm2 | ||||
| Total area | 0.78±0.83 | 0.97±0.95 | 0.58±0.62 | <.001 |
| Carotid area | 0.33±0.39 | 0.39±0.44 | 0.27±0.3 | <.001 |
| Femoral area | 0.72±0.63 | 0.84±0.7 | 0.56±0.49 | <.001 |
| Carotid stenosis | .224 | |||
| No stenosis | 8274 (99.22) | 4084 (99.43) | 4190 (99.04) | |
| ≥ 50 stenosis | 56 (0.78) | 24 (0.57) | 32 (0.96) | |
| Intracranial stenosis | .019 | |||
| No stenosis | 5933 (98.91) | 3039 (99.28) | 2894 (98.57) | |
| <50 stenosis | 68 (1.07) | 23 (0.7) | 45 (1.41) | |
| ≥ 50 stenosis | 2 (0.02) | 1 (0.02) | 1 (0.02) | |
| Ankle-brachial index | <.001 | |||
| Nonpathological | 7338 (89) | 3691 (90.58) | 3647 (87.62) | |
| ABI>1.4 | 59 (0.64) | 49 (1.22) | 10 (0.13) | |
| ABI ≤ 0.9 | 930 (10.36) | 365 (8.2) | 565 (12.25) | |
| Abdominal aorta | NA | |||
| Normal | 1108 (98.24) | 1108 (98.24) | NA | |
| Aneurysm | 17 (1.76) | 17 (1.76) | NA | |
ABI, ankle-brachial index; EDV, end diastolic velocity; ICA, internal carotid artery; NA, not applicable; PSV, peak systolic velocity; PSVR, ICA PSV to common carotid ratio.
Values are shown as No. (%) for qualitative variables and as mean±standard deviation for normally distributed quantitative variables. Sample weights were applied in the analysis. A≥50 carotid stenosis was defined as ICA PSV≥125 cm/s, PSVR≥2.0 and EDV≥40 cm/s. A near occlusion stenosis was defined as a variable (high, low or undetectable) ICA PSV and a plaque estimate> 95. ≥50 and <50 intracranial stenosis were defined as PSV≥155/≥120 cm/s (anterior cerebral artery);≥220/≥155 cm/s (middle cerebral artery);≥145/≥100 cm/s (posterior cerebral artery), respectively. Abdominal aorta aneurysm was defined as abdominal aorta diameter≥3 cm.
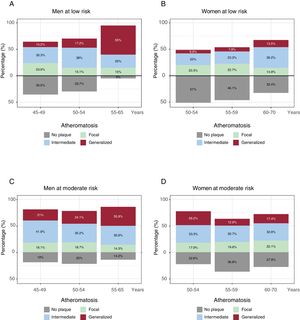
The relationship between subclinical atheromatosis burden and age is shown in figure 4. Generalized atheromatosis increased with age in men. The increase was higher in low-risk men than in moderate-risk men, as it showed a 5.4-fold increase in men with low risk (10.2% of 45 to 49-year-olds vs 55% of 55 to 65-year-olds) and a 1.7-fold increase in men with moderate risk (21% of 45 to 49-year-olds vs 35.9% of 55 to 65-year-olds). A similar tendency was observed in women with low risk (6.6% of 50 to 54-year-olds vs 13.5% of 60 to 70-year-olds). In contrast, a reduction with increased age was observed in women with moderate risk (26.2% of 50 to 54-year-olds vs 17.4% of 60 to 70-year-olds).
Presence of subclinical atheromatosis according to traditional risk equations. A-D: atheromatosis presence and burden according to the European 10-year risk SCORE stratified by sex and age. Focal atheromatosis was defined as 1 territory out of 12 with atheroma plaque, intermediate as 2-3 territories, and generalized as> 3 territories with atheroma plaque.
Atheroma plaque mean area was higher in men (0.97cm2 men vs 0.58cm2 women; P <.001). Plaques were larger in femoral arteries (0.72 femoral vs 0.33 carotid; P <.001) (table 4). Plaque area increased according to the prevalence of cardiovascular risk factors and the number of territories with plaque (figure 5).
Peripheral arterial disease was found in 11% of participants. Of these, 10.4% had an ankle-brachial index ≤ 0.9, suggestive of stenosis and 0.64% an ankle-braquial index> 1.4, indicative of vascular stiffness. Stenosis was more frequent in women, whereas vascular stiffness was more prevalent in men (12.3% vs 8.2% and 1.2% vs 0.1%; P=.006, respectively). Transcranial ultrasound revealed 68 cases (1.1%) with <50% stenosis and 2 cases with ≥ 50%. Finally, abdominal aortic aneurysm screening in men older than 60 years revealed 17 cases (1.8%) (table 4).
DISCUSSIONThe most important findings of the present study are: a) the ILERVAS study identified several previously undiagnosed chronic diseases, such as dyslipidemia, hypertension, diabetes, obesity, and kidney disease in an otherwise healthy general population; b) subclinical atheromatosis was highly prevalent in otherwise healthy middle-aged population, and the most frequent localization was common femoral, followed by carotid bifurcation and internal carotid; c) one third of the participants, classified as low-to-moderate cardiovascular risk, had intermediate atheromatosis (2-3 territories with plaque out of 12), and one fifth had generalized atheromatosis (> 3 territories); and d) total plaque area rises as the number of cardiovascular risk factors increase.
The ILERVAS study is the first study that exhaustively and globally screened atheromatosis, occult chronic kidney disease, diabetes, and prediabetes in a middle-aged asymptomatic population with at least 1 CV risk factor, representative of the general population. The mobile unit travelled across the entire province of Lleida, recruiting participants from all municipalities to obtain a representative sample of the whole population with the inclusion criteria.
Few cohort studies have investigated the prevalence and atheromatosis burden using vascular ultrasound across multiple vascular sites in middle-aged participants without a previous cardiovascular event. On the contrary, similar Spanish studies focused on atheromatosis, such as PESA and AWHS, only recruited employees of the Santander Bank in Madrid (Madrid, Spain) or General Motors automobile assembly plant in Figueruelas (Zaragoza, Aragón, Spain), respectively. Thus, a clear bias on age and even sex can affect the results of those cohorts. Our data showed a 71.4% of prevalence of subclinical atheromatosis in middle-aged population, a higher prevalence compared with the PESA study where 63% of participants showed atheromatosis.9 The higher prevalence of atheromatosis in our study could be due to the fact that our cohort is 12 years older in average, although women are more represented in our cohort (51% ILERVAS study vs 37% PESA study). On the other hand, the AWHS (Aragon Workers’ Health Study), with younger participants, but only men, showed a prevalence of 72%,14 slightly lower than the 79.7% present in our subpopulation of men, probably due to their older age.
Similar to PESA and AWHS14 studies, femoral artery was the most frequent affected vascular site; possibly due to shear stress and disturbed blood flow caused by the formal artery curvature.15 In femoral arteries, the association of atheromatosis with risk factors was stronger than in carotid or coronary arteries.14 Femoral atheroma plaques had similar predictive value for cardiovascular events; and increased plaque burden, with plaques in both carotid and femoral arteries increased the cardiovascular risk further.16 Thus, femoral artery evaluation could improve risk assessment.
Our work also confirmed the lack of correlation between the estimated 10-year cardiovascular risk assessment with clinical data and the actual presence subclinical atheromatosis.9,17,18 Up to 58% of individuals considered being at low risk in the PESA study and 57% in the AWHS study had atheromatosis. By contrast, the ILERVAS cohort showed that 71.4% of our low-to-moderate participants had subclinical atheromatosis. Regarding atheromatosis burden, one third of participants classified as low-to-moderate risk had intermediate atheromatosis (2-3 territories with plaque out of 12), and one fifth had generalized atheromatosis (> 3 territories). Similar results were obtained in the PESA study, where one third of participants with low risk had 2 sites affected. Traditionally, the absence of conventional CVRF was considered a reliable indicator of low atheromatosis risk. However, PESA investigators identified multiterritorial atheromatosis in nearly 30% of participants without CVRF. Therefore, atheromatosis is associated with factors not included in standard risk scales, an aspect that requires further investigation.19
In agreement with previous studies, the ILERVAS study revealed that atheroma plaque area was almost double in men than in women.20,21 The prevalence of atherosclerosis in carotid arteries had been reported to be low in women before menopause, and subsequently becoming similar to men.22 One possible explanation is that women benefit from protective effects of ovarian hormones and men possibly engage in more health-damaging behaviours such as smoking.23 Additionally, a higher area was observed in femoral arteries in both sexs, although plaque presence was more frequent in the femoral arteries in men, but in the carotid arteries in women. Similar results were obtained in the PESA study using 3D vascular ultrasound.21
The prevalence of intracranial stenosis was extremely low when compared with the Barcelona-AsiA study24 (1.72% ILERVAS study vs 8.6% Barcelona-AsiA study). Barcelona-AsiA cohort was an older moderate-to-high cardiovascular risk population from an urban ambient. Similar comparisons with Asian populations also showed a dramatic reduction when comparing elderly urban populations25 to younger rural,26 with prevalence dropping from 24.5% to 4.7%.
The 45.2% of participants showed a high risk of obstructive sleep apnoea (OSA) according to the Berlin questionnaire. This prevalence is higher than the previously reported in general population27–29. This difference could be due to older participants with at least one CVRF. OSA is a frequent sleep disorder associated with poor quality of life, cardiometabolic alterations, and high blood pressure that contributes to the pathogenesis of cardiovascular disease30.
Limitations and strenghthsOur study has several limitations that need to be pointed out. First, blood biochemical parameters were obtained by dried blood spot tests and urine reagent strips were used to determine albumin-to-creatinine ratio. Importantly, these systems are highly validated clinical chemistry methods which results highly correlate to well standardized laboratory methods.31-33 Second, lipid profile was evaluated only in participants in whom total cholesterol was ≥ 200mg/dL after 6hours fasting or when total cholesterol was ≥ 250mg/dL regardless of fasting hours. Although this is not the standard recommendation, recent guidelines propose that in adults ≥ 20 years old and without lipid lowering therapy (the 81.4% of our cohort), measurement of either fasting or nonfasting lipid profile is effective in estimating atherosclerotic cardiovascular risk and recording LDL-C.34 Third, the duration of physical activity and food consumption was self-reported by participants. This study did not use wearable activity devices or a weighed dietary record to obtain a detailed dietary pattern. Although they are internationally used and validated questionnaires to characterize lifestyle parameters, the IPAQ questionnaire has a demonstrated systematic bias toward underestimation of physical activity-related energy expenditure at higher levels of physical activity.35 On the contrary, 14-item MEDAS questionnaire had a high concordance with a full-length food frequency questionnaire. Therefore, MEDAS is a useful tool in clinical trials and in practice to assess adherence to Mediterranean diet.36 Forth, respiratory parameters and transcranial ultrasound were collected in a subsample of participants (6209 and 6301 participants, respectively). Finally, the results from the ILERVAS study cannot be generalized to the entire population, since participants were Spanish middle-aged people with low-to-moderate cardiovascular risk. Therefore, more studies with representation of all age groups are needed to confirm our results.
In contrast, our study has several strengths. First, the study population was randomized, and a stratified sampling was performed from primary care records to reduce selection bias, and to obtain a representative cohort of the entire province. Second, the mobile unit travelled across the entire province of Lleida. This allowed us to cover isolated and/or difficult to access villages usually not included in cohort studies, and to perform ultrasounds by the same itinerant team, avoiding interoperator bias. Third, participants received a combined screening of subclinical atheromatosis, kidney disease, sleep disorders, and pulmonary function. The protocol was designed and supervised by several medical and nurse specialists. The ILERVAS team is a multidisciplinary team formed by vascular imaging specialists, nephrologists, primary care physicians, neumologists, neurologists, nurses, internists, endocrinologist, and epidemiologists. Finally, this project has an added value in terms of primary prevention. ILERVAS identified several previously undiagnosed chronic subclinical diseases, such as atheromatosis, prediabetes, diabetes, occult kidney disease, respiratory disorders, and even aortic aneurisms in a low-to-moderate cardiovascular risk population of the North-East region of Spain.
CONCLUSIONSIn conclusion, our mobile unit detected several previously undiagnosed chronic diseases, such as dyslipidemia (21.1%), hypertension (15.3%), kidney disease (15.4%), obesity (10.6%), and diabetes (2.3%) in a cohort of middle-aged participants without a previous cardiovascular event. Subclinical atheromatosis was highly prevalent (71.4%) in this cohort, classified as low-to-moderate cardiovascular risk by current algorithms. The most frequent localization of atheroma plaques was common femoral, followed by carotid bifurcation and internal carotid, with one third of the cohort showing intermediate atheromatosis (2-3 territories with atheroma plaque), and one fifth generalized atheromatosis (> 3 territories). Atheroma plaque area was larger in femoral than in carotid arteries, and it increased with the number of CVRF and the number of affected vascular territories. Men showed larger plaques compared with women.
- -
Atheromatous cardiovascular disease is the leading cause of mortality and disability in most countries.
- -
The association of atheroma plaque with cardiovascular events has been extensively confirmed.
- -
Although atheromatosis-related adverse events are frequently developed even in low-to-moderate cardiovascular risk individuals, only a few studies have been conducted in this population.
- -
The ILERVAS study is a randomized representative cohort of a general Spanish population that exhaustively and globally screens atheromatosis in a middle-aged asymptomatic population with at least one CVRF.
- -
Data analysis revealed that one third had intermediate atheromatosis (2-3 territories with atheroma plaque), and one fifth had generalized atheromatosis (> 3 territories).
- -
Atheroma plaque area was larger in femoral than in carotid arteries; it increased with the number of CVRF and the number of affected vascular territories; and men showed larger plaques compared with women.
This work was supported by grants from the Diputació de Lleida, Instituto de Salud Carlos III (RETIC RD16/0009/0011) and Ministerio de Ciencia, Innovación y Universidades (IJC2018-037792-I).
CONFLICTS OF INTERESTNone.
The authors would like to thank Virtudes María, Marta Elias, Teresa Molí, Cristina Domínguez, Noemí Nova, Alba Prunera, Núria Sans, Meritxell Soria, Francesc Pons, Rebeca Senar, Pau Guix, Fundació Renal Jaume Arnó, and the primary care teams of the province of Lleida for recruiting participants and their efforts in the accurate development of the ILERVAS project. Samples were obtained with support from IRBLleida Biobank (B.0000682) and Plataforma Biobancos PT17/0015/0027.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.09.015