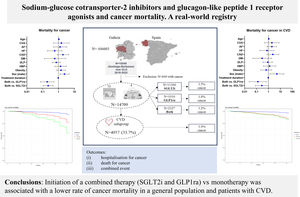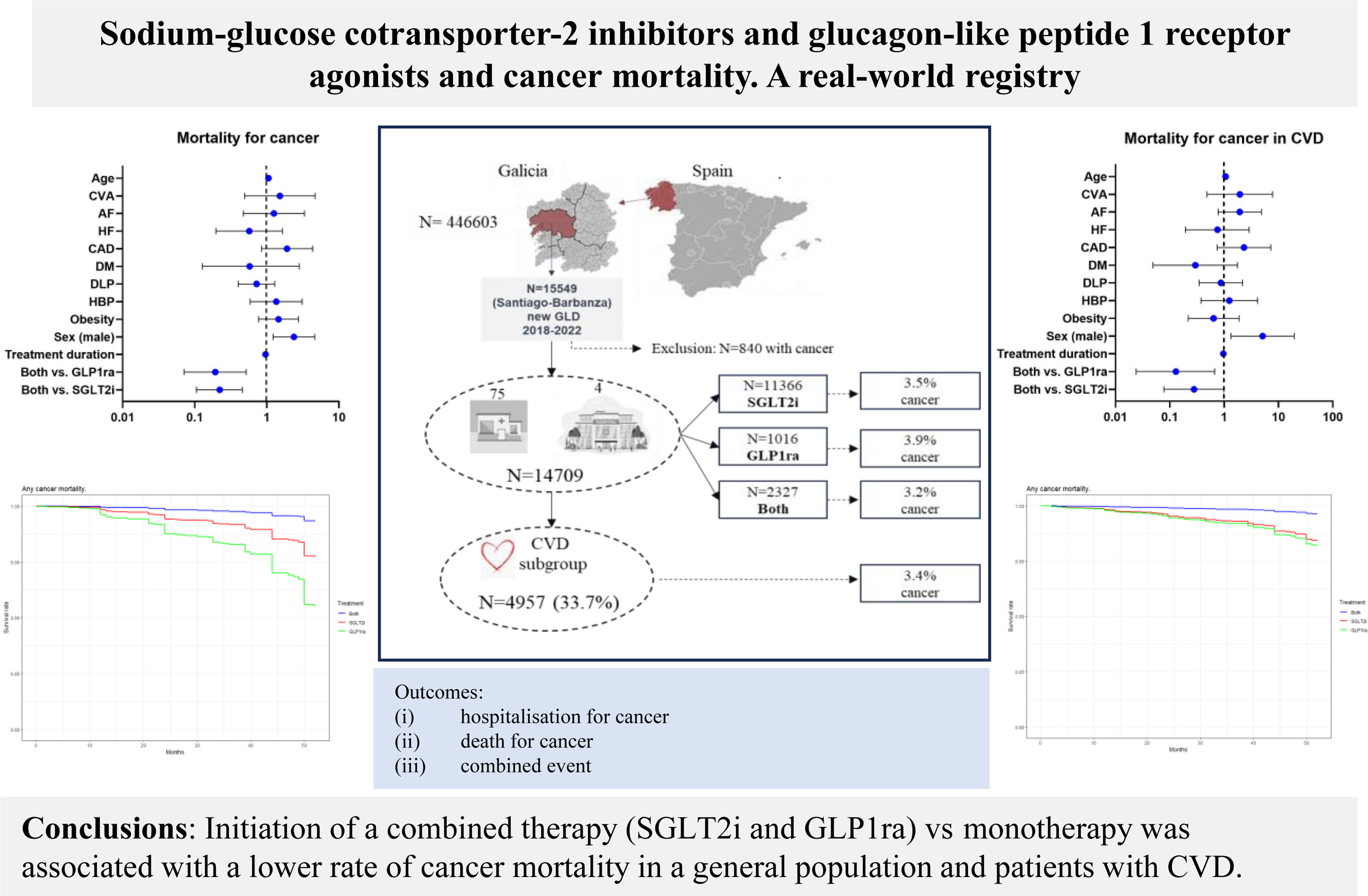
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1ra) reduce cardiovascular events through different mechanisms, but their association with cancer remains unclear. The aim of this study was to compare the effect of combined treatment (SGLT2i and GLP1ra) and monotherapy (SGLT2i or GLP1ra) on hospitalization and/or death from cancer in a general population and a subgroup of patients with cardiovascular disease (CVD).
MethodsWe conducted a nonconcurrent observational prospective study of patients prescribed SGLT2i, GLP1ra, or both. Multinomial propensity scores were performed in the entire population and in a subgroup of patients with CVD. A multivariate Cox regression analysis was used to determine the hazard ratio (HR) for age, sex, risk factors, and treatment for each outcome.
ResultsWe included 14 709 patients (11366 with SGLT2i, 1016 with GLP1ra, and 2327 with both treatments) from treatment initiation. Diabetes was present in 97% of the patients. The subgroup with CVD included 4957 (33.7%) patients. After a median of 33 months of follow-up, the risk of adverse cancer events was similar between patients with and without CVD (3.4% or 3.7%, respectively). The main risk factors for cancer mortality were male sex and age. Combined treatment and its duration reduced the risk of cancer mortality compared with monotherapy with SGLT2i or GLP1ra in the overall population (HR, 0.2216; 95%CI, 0.1106-0.4659; P<.001; and HR, 0.1928; 95%CI, 0.071-0.5219; P=.001, respectively) and in the subgroup of patients with CVD (HR, 0.2879; 95%CI, 0.0878-0.994; P<.049; and HR, 0.1329; 95%CI, 0.024-0.6768; P=.014, respectively).
ConclusionsInitiation of combined therapy (SGLT2i and GLP1ra) vs monotherapy with SGLT2i or GLP1ra was associated with a lower risk of cancer mortality, mostly in diabetic patients with or without CVD. Although clinical trials are needed, these results might be explained by the complementary mechanisms of these drugs, including their antiproliferative, anti-inflammatory, and metabolic effects. Future clinical trials and mechanistic studies will clarify the possible role of these drugs in carcinogenesis.
Keywords
Obesity is a risk factor for several major cancers, including those of the breast, gastrointestinal system, kidneys, and genitourinary tract. High body fat can increase cancer mortality risk1 by 17% through mechanisms such as chronic inflammation, oxidative stress, insulin resistance, adipokine activity, epithelial-mesenchymal transition, and endoplasmic reticulum stress.2,3 These mechanisms are common with diabetes, which can also increase cancer mortality.4
Glucagon-like peptide-1 receptor agonists (GLP1ra) and sodium-glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated reductions in adipose tissue5,6 and improvements in metabolism, including normalization of blood glucose levels.7
Some observational studies have confirmed that GLP1ra, compared with other antidiabetic drugs, reduces liver events8 in patients with diabetes, and decreases the risk of colorectal9 and prostate cancer10 in patients with obesity or overweight. Through the PI3K/Akt/mTOR signal transduction pathway, GLP1ra may induce apoptosis of cancer cells and inhibit their proliferation and migration.11 Similar antiproliferative activity has been described SGLT2i,12,13 and GLP1ra.14 Its role in glucose uptake reduction by cancer cells suggested attractive and protective mechanisms against their proliferation.15 Observational studies of diabetic patients with cancer and SGLT2i treatment have also shown improvements in overall survival.16 SGLT2i can reduce clinical events in patients with cardiac dysfunction caused by cancer therapy.17 While these results suggest a damaging mechanism on cancer cells, a protective mechanism may improve the survival of heart cells against antineoplastic treatments.
A real-world cohort suggested higher thyroid cancer risk in patients with GLP1ra intake18 and bladder cancer risk in patients under SGLT2i treatment.19 These contradictory data might be explained by confounding factors.20
The 2 therapies are complementary. While GLP1ra increases insulin secretion and activity, SGLT2i decreases plasma glucose reabsorption in the proximal tubule.21 Cardiovascular disease (CVD) and cancer are the main causes of death worldwide and glucose metabolism and obesity play important roles. Our previous data suggested the benefit of combined therapy with SGLT2i and GLP1ra for heart failure (HF) and all-cause mortality.22 However, there is no evidence regarding cancer adverse events.
METHODSData sourcesRegisters were codified without any personal data, and informed consent was not required after approval by the Clinical Research Ethics Committee of Galicia (code: 2023/239) according to General Data Protection Regulation.
The database was designed using primary care and hospital records that provide health care coverage to 446 603 inhabitants. Data were obtained through “big data” technologies from the electronic medical histories of the population, as previously described.23
Inclusion criteria consisted of all patients in our health area aged 18 years or older who were taking SGLT2i, GLP1ra, or both at the same time from January 2018 to 30th June 2022 and who were followed-up until October 2022.
Exclusion criteria consisted of a history of any cancer or electronic records outside the public health system.
Clinical variables comprised demographic data (sex and age), personal clinical history including cardiovascular risk factors (high blood pressure, diabetes mellitus, dyslipidemia, obesity or overweight); CVD conditions such as atrial fibrillation (AF), HF, coronary artery disease (CAD), peripheral arterial disease, or stroke; and treatment duration. All diseases were classified according to the International Classification of Diseases, 10th Revision (ICD-10).
Cancer classification was defined according to organs and systems: blood and hematology, brain and nervous system, breast, digestive, endocrine, head and neck, locomotive, renal and genitourinary, respiratory, skin. Rare cancers not fitting in these classifications were included in the category of “others”.
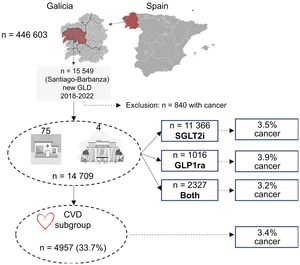
Study designThis nonconcurrent observational study included patients from a single Spanish health care area (Santiago de Compostela - Barbanza). This area consists of 4 public hospitals and 75 health care centers. Patients were followed up from the index date of glucose-lowering drug (GLD) initiation until October 31, 2022, or death (figure 1), regardless of whether the cause of death was cancer or a noncancer cause, as recommended in a previous statistical study.24
OutcomesThe 3 primary outcomes were: a) hospitalization for any cancer, b) death from any cancer, and c) hospitalization and/or death from any cancer, according to ICD-10 codes in the included population and the subgroup of patients with CVD. Cancer hospitalization included all hospitalizations related to any cancer, including first cancer diagnosis and either urgent or elective hospitalizations; we did not consider hospitalizations in which the main cause was other diagnoses (eg, gastrointestinal bleeding) without any competing risk. Death from any cancer included both in-hospital and out-of-hospital mortality related to any cancer. Cancer events included any of the 3 primary outcomes.
We obtained epidemiological and administrative data from the health care database and electronic clinical records using ICD-10 codes for cancer-related information and hospitalizations, and International Classification of Primary Care (ICPC-2) codes for personal medical histories. Mortality causes were incorporated into the health care database from in-hospital and out-of-hospital mortality records. Cancer information was obtained from electronic clinical records, documented with ICPC-2 codes in primary care or ICD-10 codes for hospitalized patients, and from ambulatory assistance in the oncology and radiotherapy departments.25
Statistical analysisDescriptive dataCategorical variables are reported as percentages (%). Quantitative variables are expressed as median [interquartile range] or mean±standard deviation, according to the normality of their distribution, which was checked with the Kolmogorov-Smirnov test.
Differences among groupsAfter dividing the population according to GLD therapy (SGLT2i and/or GLP1ra), differences in the main included variables were determined by the F-test or Wilcoxon test in categorical or continuous variables, respectively.
Propensity score and predictors for eventsWe used Toolkit for weighting and analysis of nonequivalent groups, specifically TWANG version 2.6 in R, to perform multinomial propensity score weighting to manage the 3 treatment groups (SGLT2i, GLP1ra, and both treatments). The adjustment included the following variables among the three groups: sex, age, obesity, diabetes mellitus, high blood pressure, dyslipidemia, CAD, HF, AF, and stroke.
Balance statistics were assessed using the standardized mean difference, also known as the absolute standardized bias or effect size, and the Kolmogorov-Smirnov statistic. A threshold value of 0.1 for the standardized mean difference was considered to evaluate balance among the groups.26 This methodology was applied similarly for patients with CVD.
Event-free survivalMultivariate Cox regression analysis was conducted to assess the impact of several variables on hospitalization and/or death from any cancer. The variables included in the analysis were sex, age, obesity, diabetes mellitus, high blood pressure, dyslipidemia, CAD, HF, AF, stroke, treatment type, and treatment duration. Hazard ratios (HRs) and their corresponding 95% confidence intervals (95%CIs) were calculated to quantify the effect of each variable. These results were visualized using forest plots and survival plots.
The analyses were performed using R version 4.2.3 statistical package. The same methodology was applied to analyze patients with CVD, ensuring comprehensive evaluation of the impact of these variables among different patient groups.
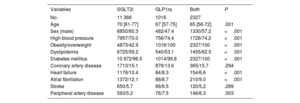
RESULTSClinical characteristics of included patientsOut of 15 549 patients who were taking SGLT2i or GLP1ra, or both, 14 709 had no history of cancer. The population was predominantly male (58.9%), with a high prevalence of obesity (55.9%), high blood pressure (71%), dyslipidemia (59.3%), and diabetes mellitus (97.3%). In addition, 15.1% had CAD, 9.6% had HF, 11.4% had AF, 5.7% had had a stroke, and 5.5% had peripheral arterial disease (PAD). The median age of the population was 69 [range, 60-76] years, and the median treatment duration was 14 [range, 5-35] months. Among the included patients, 33.7% (4957 patients) had some form of cardiovascular disease (CAD, HF, AF, stroke, and/or PAD).
Multinomial propensity score analysis was conducted after ensuring the absence of statistically significant differences in clinical characteristics among the treatment groups (table 1). SGLT2i was the most frequently prescribed drug (78.7%), followed by combined treatment with SGLT2i and GLP1ra (14.9%), and GLP1ra alone (6.4%). The mean follow-up duration was 33±months.
Clinical characteristics according to treatment
| Variables | SGLT2i | GLP1ra | Both | P |
|---|---|---|---|---|
| No. | 11 366 | 1016 | 2327 | |
| Age | 70 [61-77] | 67 [57-75] | 65 [56-72] | .001 |
| Sex (male) | 6850/60.3 | 482/47.4 | 1330/57.2 | <.001 |
| High blood pressure | 7957/70.0 | 756/74.4 | 1726/74.2 | <.001 |
| Obesity/overweight | 4873/42.9 | 1016/100 | 2327/100 | <.001 |
| Dyslipidemia | 6725/59.2 | 540/53.1 | 1455/62.5 | <.001 |
| Diabetes mellitus | 10 972/96.5 | 1014/99.8 | 2327/100 | <.001 |
| Coronary artery disease | 1713/15.1 | 878/13.6 | 365/15.7 | .294 |
| Heart failure | 1178/10.4 | 84/8.3 | 154/6.6 | <.001 |
| Atrial fibrillation | 1372/12.1 | 88/8.7 | 210/9.0 | <.001 |
| Stroke | 650/5.7 | 66/6.5 | 120/5.2 | .289 |
| Peripheral artery disease | 593/5.2 | 76/7.5 | 146/6.3 | .003 |
The data are expressed as No./% or median [interquartile range].
GLP1ra, glucagon-like peptide 1 receptor agonists; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
The most commonly prescribed SGLT2i medications were empagliflozin (n=6056), dapagliflozin (n=5802), and canagliflozin (n=1778). Only 24 patients were treated with ertugliflozin. Among GLP-1 receptor agonists, the most prevalent were dulaglutide (n=1864), semaglutide (n=934), liraglutide (n=400), and exenatide (n=214). Only 24 patients were treated with lixisenatide.
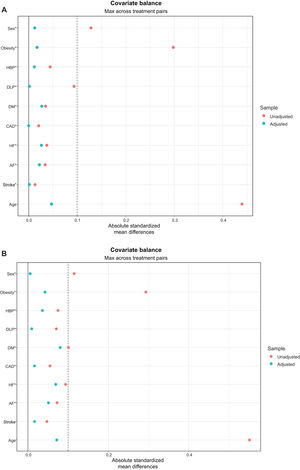
Multinomial propensity scoreThe means, standard deviations, and standardized effect sizes between paired groups were balanced. The absolute mean differences of all included variables were less than 0.1. The covariate balance before and after adjustment was depicted in love plots for all included patients and those with CVD (figure 2A and 2B, respectively).
Love plots display standardized mean differences for all the covariates in the population (A) or patients with CVD (B) before and after adjustment by multinomial propensity score. AF, atrial fibrillation; DM, diabetes mellitus; CAD, coronary artery disease; CVD, cardiovascular disease; DLP, dyslipidemia; HBP, high blood pressure; HF, heart failure.
Adverse cancer events, including hospitalization for cancer, cancer mortality, and combined events, were reported in 514 out of 14 709 patients (3.5%). The most prevalent types of cancer were digestive (38%) and renal-genitourinary (24%). Cancer of the respiratory system accounted for 11%, breast cancer for 6%, and other types were less frequent.
Specifically, hospitalization for cancer occurred in 3.2%, 3.9%, and 3.1% of patients treated with SGLT2i, GLP1ra, and combined therapy, respectively. Cancer mortality rates were 0.9%, 1.0%, and 0.4% in patients treated with SGLT2i, GLP1ra, and combined therapy, respectively. Combined hospitalization and/or death from cancer occurred in 3.5%, 3.9%, and 3.2% of patients treated with SGLT2i, GLP1ra, and combined therapy, respectively.
When considering only patients with previous CVD, the percentages of outcomes observed were: 3.3%, 4.1%, and 3.2% for hospitalization for cancer; 0.8%, 1.6%, and 0.4% for cancer mortality; and 3.8%, 4.1%, and 3.2% for combined hospitalization and/or death from cancer in patients treated with SGLT2i, GLP1ra, and combined therapy, respectively.
The percentage of cancer events was similar between patients with and without CVD (3.4% or 3.7%, respectively).
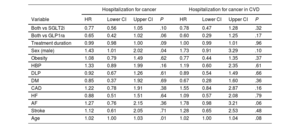
The multivariate Cox regression analysis revealed that male sex and age were associated with an increased risk of cancer hospitalization (HR, 1.4439; 95%CI, 1.193-1.735; and HR, 1.0329; 95%CI, 1.020-1.0438, respectively). However, the combined treatment and its duration showed a reduction in risk, although without statistical significance (table 2). These associations were not observed in the subgroup of patients with CVD.
Multivariate Cox regression analysis. Hospitalization for cancer
| Hospitalization for cancer | Hospitalization for cancer in CVD | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | Lower CI | Upper CI | P | HR | Lower CI | Upper CI | P |
| Both vs SGLT2i | 0.77 | 0.56 | 1.05 | .10 | 0.78 | 0.47 | 1.28 | .32 |
| Both vs GLP1ra | 0.65 | 0.42 | 1.02 | .06 | 0.60 | 0.29 | 1.25 | .17 |
| Treatment duration | 0.99 | 0.98 | 1.00 | .09 | 1.00 | 0.99 | 1.01 | .96 |
| Sex (male) | 1.43 | 1.01 | 2.02 | .04 | 1.73 | 0.91 | 3.29 | .10 |
| Obesity | 1.08 | 0.79 | 1.49 | .62 | 0.77 | 0.44 | 1.35 | .37 |
| HBP | 1.33 | 0.89 | 1.99 | .16 | 1.19 | 0.60 | 2.35 | .61 |
| DLP | 0.92 | 0.67 | 1.26 | .61 | 0.89 | 0.54 | 1.49 | .66 |
| DM | 0.85 | 0.37 | 1.92 | .69 | 0.67 | 0.28 | 1.60 | .36 |
| CAD | 1.22 | 0.78 | 1.91 | .38 | 1.55 | 0.84 | 2.87 | .16 |
| HF | 0.88 | 0.51 | 1.51 | .64 | 1.09 | 0.57 | 2.08 | .79 |
| AF | 1.27 | 0.76 | 2.15 | .36 | 1.78 | 0.98 | 3.21 | .06 |
| Stroke | 1.12 | 0.61 | 2.05 | .71 | 1.28 | 0.65 | 2.53 | .48 |
| Age | 1.02 | 1.00 | 1.03 | .01 | 1.02 | 1.00 | 1.04 | .08 |
AF, atrial fibrillation; CAD, coronary artery disease; CVD, cardiovascular disease; CI, confidence interval; DLP, dyslipidemia; DM, diabetes mellitus; HBP, high blood pressure; HF, heart failure; GLP1ra, glucagon-like peptide 1 receptor agonists; HR, hazard ratio; PAD, peripheral artery disease; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
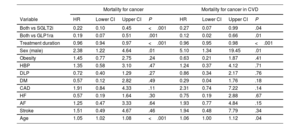
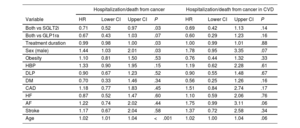
For cancer mortality, male sex and age were also identified as significant risk factors (HR, 2.3488; 95%CI, 1.2327-4.645, and HR, 1.054; 95%CI, 1.024-1.085, respectively). Conversely, a lower risk was observed with combined treatment and its duration compared with SGLT2i or GLP1ra alone (table 3) in the overall population (HR, 0.222; 95%CI, 0.1106-0.4596; P<.001; or HR, 0.193; 95%CI, 0.071-0.5219; P=.001, respectively) and in the subgroup of patients with CVD (HR, 0.2879; 95%CI, 0.0878-0.994; P<.049; or HR, 0.1329; 95%CI, 0.024-0.6768; P=.014, respectively). Similar results were observed for hospitalization and/or death from any cancer cause (table 4).
Multivariate Cox regression analysis. Mortality from cancer
| Mortality for cancer | Mortality for cancer in CVD | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | Lower CI | Upper CI | P | HR | Lower CI | Upper CI | P |
| Both vs SGLT2i | 0.22 | 0.10 | 0.45 | <.001 | 0.27 | 0.07 | 0.99 | .04 |
| Both vs GLP1ra | 0.19 | 0.07 | 0.51 | .001 | 0.12 | 0.02 | 0.66 | .01 |
| Treatment duration | 0.96 | 0.94 | 0.97 | <.001 | 0.96 | 0.95 | 0.98 | <.001 |
| Sex (male) | 2.38 | 1.22 | 4.64 | .01 | 5.10 | 1.34 | 19.45 | .01 |
| Obesity | 1.45 | 0.77 | 2.75 | .24 | 0.63 | 0.21 | 1.87 | .41 |
| HBP | 1.35 | 0.58 | 3.10 | .47 | 1.24 | 0.37 | 4.12 | .71 |
| DLP | 0.72 | 0.40 | 1.29 | .27 | 0.86 | 0.34 | 2.17 | .76 |
| DM | 0.57 | 0.12 | 2.82 | .49 | 0.29 | 0.04 | 1.76 | .18 |
| CAD | 1.91 | 0.84 | 4.33 | .11 | 2.31 | 0.74 | 7.22 | .14 |
| HF | 0.57 | 0.19 | 1.64 | .30 | 0.75 | 0.19 | 2.88 | .67 |
| AF | 1.25 | 0.47 | 3.33 | .64 | 1.93 | 0.77 | 4.84 | .15 |
| Stroke | 1.51 | 0.49 | 4.67 | .46 | 1.94 | 0.48 | 7.79 | .34 |
| Age | 1.05 | 1.02 | 1.08 | <.001 | 1.06 | 1.00 | 1.12 | .04 |
95%CI, 95% confidence interval; AF, atrial fibrillation; CAD, coronary artery disease; CVD, cardiovascular disease; DM, diabetes mellitus; DLP, dyslipidemia; GLP1ra, glucagon-like peptide 1 receptor agonists; HBP, high blood pressure; HF, heart failure; HR, hazard ratio; PAD, peripheral artery disease; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Multivariate Cox regression analysis. Hospitalization and/or mortality for cancer
| Hospitalization/death from cancer | Hospitalization/death from cancer in CVD | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | Lower CI | Upper CI | P | HR | Lower CI | Upper CI | P |
| Both vs SGLT2i | 0.71 | 0.52 | 0.97 | .03 | 0.69 | 0.42 | 1.13 | .14 |
| Both vs GLP1ra | 0.67 | 0.43 | 1.03 | .07 | 0.60 | 0.29 | 1.23 | .16 |
| Treatment duration | 0.99 | 0.98 | 1.00 | .03 | 1.00 | 0.99 | 1.01 | .88 |
| Sex (male) | 1.44 | 1.03 | 2.01 | .03 | 1.78 | 0.95 | 3.35 | .07 |
| Obesity | 1.10 | 0.81 | 1.50 | .53 | 0.76 | 0.44 | 1.32 | .33 |
| HBP | 1.33 | 0.90 | 1.95 | .15 | 1.19 | 0.62 | 2.28 | .61 |
| DLP | 0.90 | 0.67 | 1.23 | .52 | 0.90 | 0.55 | 1.48 | .67 |
| DM | 0.70 | 0.33 | 1.46 | .34 | 0.56 | 0.25 | 1.26 | .16 |
| CAD | 1.18 | 0.77 | 1.83 | .45 | 1.51 | 0.84 | 2.74 | .17 |
| HF | 0.87 | 0.52 | 1.47 | .60 | 1.10 | 0.59 | 2.06 | .76 |
| AF | 1.22 | 0.74 | 2.02 | .44 | 1.75 | 0.99 | 3.11 | .06 |
| Stroke | 1.17 | 0.67 | 2.04 | .58 | 1.37 | 0.72 | 2.58 | .34 |
| Age | 1.02 | 1.01 | 1.04 | <.001 | 1.02 | 1.00 | 1.04 | .06 |
95%CI, 95% confidence interval; AF, atrial fibrillation; CAD, coronary artery disease; CVD, cardiovascular disease; DM, diabetes mellitus; DLP, dyslipidemia; GLP1ra, glucagon-like peptide 1 receptor agonists; HBP, high blood pressure; HF, heart failure; HR, hazard ratio; PAD, peripheral artery disease; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
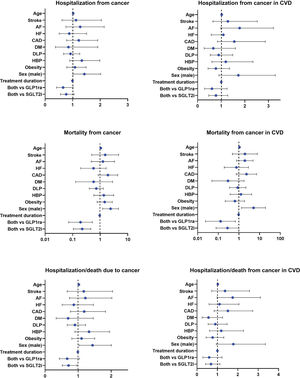
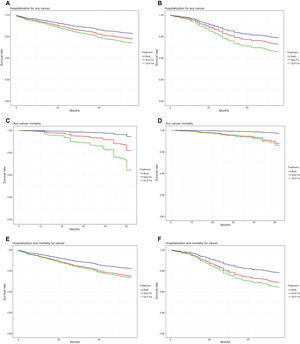
The results of the multivariate Cox regression analysis were visualized using forest plots (figure 3). Survival analysis indicated that there was no significant reduction in the rate of hospitalization for any cancer among patients receiving combined therapy compared with those receiving SGLT2i or GLP1ra alone, both in the general population (figure 4A) and in the subgroup of patients with stroke (figure 4B).
Forest plots depict the hazard ratio and 95% confidence interval of each factor for hospitalization for cancer in the population (A) and patients with CVD (B), for cancer mortality in the population (C) and patients with CVD (D) and for hospitalization/death from cancer in the population (E) and patients with CVD (F). AF, atrial fibrillation; CAD, coronary artery disease; CVD, cardiovascular disease; DM, diabetes mellitus; DLP, dyslipidemia; GLP1ra, glucagon-like peptide 1 receptor agonists; HBP, high blood pressure; HF, heart failure; HR, hazard ratio; PAD, peripheral artery disease; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Cox regression survival curve on SGLT2i, GLP1ra, and combined therapy groups for hospitalization for cancer in the population (A) and patients with CVD (B), for cancer mortality in the population (C) and patients with CVD (D) and for hospitalization/death from cancer in the population (E) and patients with CVD (F). GLP1ra, glucagon-like peptide 1 receptor agonists; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
However, a higher survival rate for any cancer was observed among patients receiving combined therapy compared with those receiving SGLT2i or GLP1ra alone, both in the general population (figure 4C) and in the subgroup of patients with CVD (figure 4D).
In addition, a lower risk of hospitalization and/or death from any cancer was observed among patients receiving combined therapy compared with those receiving SGLT2i alone in the general population (figure 4E). This effect was not observed in the subgroup of patients with CVD (figure 4G).
DISCUSSIONMain findingsIn this large real-world population registry of patients without prior cancer who were receiving SGLT2i, GLP1ra, or both treatments, with a median follow-up of 33 months, combined therapy involving SGLT2i or GLP1ra was associated with a reduced risk of cancer mortality in both the general population and in the subgroup of patients with CVD, comprising 33.7% of the total population. The incidence of adverse cancer events with these treatments was 3.5%.
Gastrointestinal cancers accounted for the highest mortality (45%), followed by respiratory (22%) and renal/genitourinary cancers (14%), with no significant differences observed among the therapy groups. Digestive cancers were the leading cause of death in patients receiving SGLT2i (46%), GLP1ra (30%), and combined therapy (50%).
Given that dapagliflozin, empagliflozin, and canagliflozin were the most commonly used SGLT2i, and dulaglutide and semaglutide were the predominant GLP-1ra medications, there may be a class effect. However, further studies are needed to clarify this observation.
Clinical implicationsTo our knowledge, this is the first study to report an association between combined therapy with SGLT2i and GLP1ra and a reduced risk of cancer mortality in a real-world registry. This finding may have implications for future clinical trials aimed at investigating the relationships between these treatments and cancer incidence and prognosis.
Cancer risk in SGLT2i clinical trialsThe leading causes of mortality worldwide are CVD and cancer. In our population, 97% of the patients had diabetes, and the most prevalent cancers were digestive, renal, and genitourinary. Diabetes is a known risk factor for cancer, possibly related to metabolic dysfunction and hyperglycemia. These factors can enhance oncogenic and inflammatory pathways.27 Therefore, the higher glucose excretion by SGLT2i treatment might reduce its availability in cancer cells, its energy source,28 and growth. This mechanism might explain the lower risk of cancer in those clinical trials with SGLT2i compared with other antidiabetic therapies.29,30 However, the higher rate of genitourinary cancer in the SGLT2i arm31,32 triggered alarms that were later resolved in a specific meta-analysis.19 In addition, glucose normalization would reduce bladder infections33 and, consequently, the risk of cancer. Observational studies have shown a higher risk of cancer in patients with atherogenic CVD34 and HF,35 which might be explained by aging, obesity, hyperactivity of the angiotensin-renin system, or inflammation.36 The modulation of these factors by drugs might reduce the cancer risk. The lower rate of cancer mortality in our population might be due to SGLT2i therapy, which regulates blood pressure, epicardial fat metabolism,7 natriuresis, glucose levels, vascular function, etc.37
Cancer risk in GLP1ra trialsMetabolic syndrome is a risk factor for several cancers, including digestive and genitourinary cancers.38 Obesity is associated with increased adiposity, leading to insulin resistance and hyperinsulinemia. The insulin/IGF-1 pathway contributes to epithelial-mesenchymal transition, tumor initiation, and propagation. High glucose levels also increase the Warburg effect, glucose uptake, lactate production, and acidification.39
This process contributes to aggressive phenotypes of tumors. The GLP1 receptor is expressed in many organs and was approved by the Food and Drug Administration to treat obesity due to its effects on weight loss and adiposity.40,41 However, it acts on the sympathetic nervous system,42,43 while angiogenesis might increase metastasis. Several randomized clinical trials or meta-analyses were conducted to demonstrate safety issues with GLP1ra,10,43,44 and its potential reduction in pancreatic cancer risk.45 The lower rate of colon cancer in diabetic populations, with and without obesity and GLP1ra treatment, suggests benefits in a weight reduction-independent manner.9 However, some observational studies report a modest incidence of thyroid cancer in patients treated with GLP1ra,46 which might be related to confounding factors.
Combined therapy SGLT2i and GLP1ra and cancer riskOur results demonstrated a lower risk of cancer mortality in patients treated with combined therapy. These results might be explained by complementary mechanisms. While SGLT2i increases urinary glucose excretion, GLP1ra improves insulin response, modulates adiposity, and reduces proinflammatory markers.47 Moreover, both drugs have an anti-inflammatory effect through modulation of the NF-kb pathway and the proinflammatory phenotype of macrophages.48,49 Previous studies have already identified these mechanisms as markers of poor prognosis in cancer.49 Other studies have also demonstrated that increased inflammatory factors raise the risk of cancer in patients with CVD,50 which might be modulated by combined therapy.
The short duration of treatment in these patients suggests rapid activity of these mechanisms, as previously described in a meta-analysis of clinical trials.51 The lack of statistical significance regarding hospitalization might suggest a stronger benefit of combined therapy in patients with more advanced stages. However, further studies are needed to elucidate this issue. Because noncancer mortality causes might be a competing risk,24 we attempted to address the issue by excluding patients who died of noncancer causes. The results were confirmed in a balanced population using multinomial propensity scores, after testing proportional hazard assumptions52 for this model, and finding that there was a nonsignificant relationship between residuals and time (supplementary data).
LimitationsWe acknowledge that our study design did not permit us to establish a definitive direct causality effect. Further randomized clinical trials are warranted to confirm these findings. Additionally, other real-world registries could investigate these associations in diverse populations treated with these medications.
Regarding information bias, it was impossible to determine the exact cause of death in some cases, which may have influenced some of our findings. In addition, we lacked data from private health care providers, but they represent a small proportion in our region. Our study included a large cohort of patients with comprehensive demographic, clinical, and prognostic information. Our population was not compared with controls not receiving GLDs or novel therapies, as the primary objective was to compare the effect of combined therapy with SGLT2i or GLP1ra on hospitalization and/or death from cancer.
A small number of patients were included in the GLP1ra group; however, we used a multinomial propensity score to adjust for the 3 study groups. Some variables were not recorded in this cohort: genetics, hormones, smoking, alcohol intake, anemia, kidney failure, physical and chemical exposures, lifestyle factors, and weight loss after treatment, although we included the most prevalent cardiometabolic risk factors for cancer. Only adverse cancer events necessitating hospitalization or resulting in mortality were considered. Therefore, patients with asymptomatic cancer or those diagnosed without hospitalization were not included. The clinical status of cancer patients (eg, undergoing treatment, stable, or receiving palliative care) or previous surgical treatments could not be documented.
CONCLUSIONSCombined therapy (SGLT2i and GLP1ra) reduced the risk of cancer mortality compared with treatment with SGLT2i or GLP1ra alone, particularly in diabetic patients, as 97% of the participants had diabetes (figure 5).
SGLT2i and GLP1ra reduce cardiovascular events through different mechanisms; however, their association with cancer remains unclear.
WHAT DOES THIS STUDY ADD?This study is the first to suggest, in a real-world registry, a lower rate of cancer mortality with combined therapy (SGLT2i and GLP1ra) compared with treatment with SGLT2i or GLP1ra alone in both the general population and patients with CVD. These findings may inform future clinical trial designs and basic mechanistic studies aimed at understanding the potential role of these drugs in carcinogenesis.
The following institutions have participated in this manuscript: CIBERCV (code: CB16/11/00226); Área Sanitaria de Compostela-Barbanza (SERGAS); University of Santiago de Compostela; Instituto de Investigación Sanitaria de Santiago de Compostela (IDIS); Fundación IDIS; Xunta de Galicia (GAIN: IN607B-2022-04).
ETHICAL CONSIDERATIONSRegisters were anonymized without requiring informed consent following approval by the Clinical Research Ethics Committee of Galicia (code: 2023/239) in accordance with the General Data Protection Regulation. Gender equity guidelines were adhered to as per SAGER guidelines.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEWe collected and analyzed all data without any contribution of artificial intelligence programs (eg, ChatGPT, or other similar software).
AUTHORS’ CONTRIBUTIONSD. García-Vega and S. Eiras conceived the presented idea. D. García-Vega, S. Eiras, and S. Cinza-Sanjurjo developed the methodology. S. Cinza-Sanjurjo and J.R. González-Juanatey supervised the design and methodology. All authors discussed the results and contributed to the final manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTERESTNo conflict of interest.
We thank the University Clinical Hospital and facility staff for providing a pseudonymized database, for all the health professionals and patients who participate in the electronic register of patients.