Keywords
This research project was financed by Sanofi-Aventis and Bristol-Myers Squibb. The analysis, however, was performed by an independent consultancy firm.
Received January 4, 2005. Accepted for publication July 1, 2005.
See editorial on pages 1377-80
INTRODUCTION
One of the main mechanisms associated with the pathogenesis of and acute coronary syndrome is the development of thrombosis overlying an atherosclerotic plaque.1,2 Platelet activation can be intense during such episodes, and is one of the factors most closely associated with cardiovascular events.3,4 Several studies have shown the effectiveness of antiplatelet therapy in the prevention of ischemic conditions, and clinical guides now recommend the early start of treatment with agents that prevent platelet aggregation. The long-term maintenance of such treatment is also advised.5 Acetylsalicylic acid is the antiplatelet agent most commonly used, but on occasion patients that receive this treatment remain at high risk of suffering coronary events both in the short and long term.6,7 Neither heparin nor glycoprotein IIb/IIIa antagonists have been shown to provide any clear clinical benefit when treatment is prolonged.5,8 However, a synergic anti-aggregant effect is achieved by combining clopidogrel (an inhibitor of adenosine diphosphate-induced platelet aggregation) with acetylsalicylic acid.9,10 Recently, the CURE study,6 which involved patients with non-ST-elevation acute coronary syndrome (NSTEACS), showed that after 1 year of treatment, patients that received clopidogrel plus standard therapy (i.e., with acetylsalicylic acid; clopidogrel+STh) were less likely to suffer cardiovascular death, acute myocardial infarction (AMI) or stroke than those treated with STh alone. Only 9.3% of patients treated with clopidogrel+STh suffered one of these events compared to 11.4% in the STh group (relative risk [RR]=0.80).6 Compared to the control group, significantly more major hemorrhages were seen in the clopidogrel+STh group (2.7% vs 3.7%; RR=1.35), although the number of patients who suffered life-threatening episodes was not significantly greater. Budaj et al11 analyzed the results of the CURE study with respect to thrombolysis in myocardial infarction (TIMI) risk scores, and showed the benefit of clopidogrel in low, medium and high risk patients. The incidence of events in the clopidogrel+STh group was 4.1% compared to 5.7% in the STh group (RR=0.71) in low risk patients, 9.8% compared to 11.4% (RR=0.85) in medium risk patients, and 15.9% compared to 20.7% (RR=0.73) in high risk patients.
In addition to the proven effectiveness of clopidogrel+ STh in the treatment of patients with NSTEACS, studies from different countries have shown clopidogrel to be cost-effective. The economic assessment of health interventions includes different techniques and procedures that can be used to compare information on the relationship between their costs and benefits. However, cost-effectiveness analysis is the most common way of assessing the economic characteristics of health interventions and can help show which provide the greatest benefit for the financial resources available.12-14
The aim of the present study was to determine the short-term and long-term cost-effectiveness of treatment with clopidogrel+STh for 1 year compared to that of STh alone in Spanish patients with NSTEACS.
PATIENTS AND METHOD
Both the short- and long-term cost-effectiveness analyses15,16 were performed from the perspective of a health system financing body. At the end of the first year of treatment with either clopidogrel+STh or STh alone, it was assumed that all patients would receive the latter only. The short-term effectiveness of the treatments was assessed in terms of the number of events (AMI, stroke or cardiovascular death) avoided; long-term effectiveness was measured as the number of life-years gained (LYG).
The Decision Model
No modeling was required for the short-term analysis; the outcomes with both treatments were analyzed at the end of the first year of treatment. As in a recent economic analysis involving 5 countries,17 all the clinical data used, as well as those referring to the consumption of health resources, were obtained directly from the CURE study.6 No additional procedures were necessary.
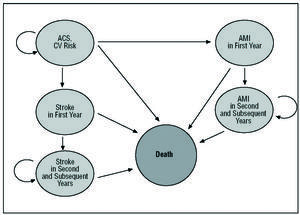
For the long-term analysis, however, a Markov model18,19 covering 6 states of health that reflect the clinical progress of patients with NSTEACS was adapted to the Spanish setting (Figure 1). Each of these health states is associated with a series of health costs and effects. The clinical progress of patients is modeled as transitions between these different health states. Each patient has a certain probability of moving from 1 health state to another. The probability of transition can vary with time and differs depending on the clinical and sociedemographic characteristics of each patient. The present patients started off in the health state of NSTEACS with a risk of suffering an event (i.e., a stroke, a non-fatal AMI or cardiovascular death, as defined in the CURE study). During this phase and throughout the following year, each received treatment with either clopidogrel+STh or STh alone. After the first year had elapsed, both groups were assumed to receive STh alone.
Figure 1. Graphical representation of the Markov model results with the health states contemplated. CV indicates cardiovascular; AMI, acute myocardial infarction; ACS, acute coronary syndrome.
From the initial phase the patients could "transit" (i.e., progress clinically) over the first year towards four of the 5 other states. Thus, at the end of the first year a patient might spend another year in the NSTEACS state (with a certain probability), suffer an AMI and transit to the "AMI in first year" state, suffer a stroke and transit to the "stroke in first year" state, or die. Once a patient has suffered an AMI or stroke in the first year the only transition possible is to death or the state of "second and subsequent years of follow-up after an AMI"/"second and subsequent years of follow-up after a stroke." Patients remain in these states during the second and following years (for a number of years or "model cycles") until they finally die.
Time Horizons and Discount Rate
In the short-term analysis the results were evaluated with respect to the mean duration of the CURE study (9 months). For the long-term analysis using the Markov model, however, the time horizon ended with the death of all the patients in the cohort. The maximum extrapolation of the model was 30 years. As indicated by guides for the economic assessment of health interventions,15,16 a discount rate of 3% was allowed for all costs and health benefits contemplated by the model that were manifested after the first year.
Result Variables
The results of the model were expressed in terms of the effectiveness of and cost differences between the clopidogrel+STh and STh regimens, and the incremental cost-effectiveness ratio (ICER). This is the ratio between the difference in the costs and the difference in the effectiveness of the 2 regimens. This effectiveness is expressed as the incremental cost per event avoided in the short-term analysis, and the incremental cost per LYG in the long-term analysis.
Effects on Health
Data on the effects of the treatment regimens on health, in terms of the reduction in the risk of suffering an event in the first year of treatment, were obtained from the CURE6 trial results. In the latter trial, the patients had a mean age of 64.2 years. Men made up 61.3%, 32.4% of whom had suffered a prior AMI. During the first year, the use of clopidogrel+STh in patients with NSTEACS was associated with an RR of suffering an event (with respect to the STh group) of 0.80 (95% CI, 0.72-0.89). The CURE results were also analyzed with respect to risk groups (i.e., high, medium and low risk patients according to their TIMI risk scores)11 and the influence of these stratifications on short-term cost-effectiveness determined. In addition, since European data on the RR of patients with acute coronary syndrome suffering an event in the long-term were available, the clinical and economic consequences of the use of clopidogrel+STh during the first and subsequent years (extrapolation) were established.
To estimate the probability of transition between health states, the long term model used empirical epidemiological data for the Swedish population.20 These data were gathered from 2 registries, one recording hospital admissions20 the other recording causes of death.21
Immediately after the initial coronary event, the risk of suffering a further event is high. This however, falls gradually until becoming constant after the first year. For this reason the distinction was made between risk during the first year and second/subsequent years. As described in the model of Lindgren et al19 the CURE results for the first year were subjected to simple logistic regression. An exponential function was used for the second and subsequent years.
Effects on Resources and Costs
This study was performed from the viewpoint of the body financing the health service, and therefore only took into account direct costs (i.e., the costs of publicly financed health resources).15 For the short-term analysis, the direct health costs associated with pharmacological treatment and patient management were obtained from the CURE study results.17 Since 232 patients in the clopidogrel+STh group suffered a serious hemorrhage compared to 170 patients in the STh group, the costs associated with treating these complications were taken into account. In the long-term analysis, the resources directly associated with the management and treatment of patients in each health state were obtained by reviewing the literature. The resources associated with hospital stay, medication, and the tests and procedures required by patients with AMI were obtained from two registries of Spanish patients with acute coronary syndrome with and without ST segment elevation (the PRAMIHO and DESCARTES registries).22,23 A group of 3 expert cardiologists was assembled to validate the model and to estimate the resource use data not available in the literature. The result of this literature review, plus the individual estimates of the three experts, allowed a mean estimate to be made of resource use.
The use of resources was divided into hospital and out-patient assistance both for events in the acute phase of disease (i.e., including the first year) and for the second and subsequent years. In all cases, medical consultations, tests and the medications administered were taken into account. In addittion, a split resource analysis was performed, separating patients with Q wave AMI from those with non-Q wave AMI, based on the proportions of these groups in the CURE study (personal communication) (40.7 and 59.3% respectively).
The unit costs of the direct health resources were taken from a Spanish setting costs database.24 Those related to medications were taken from the Catálogo de Especialidades Farmacéuticas25 (Pharmaceutical Specialities Catalogue) for 2003 (costs in euros).
Sensitivity Analysis
Univariate sensitivity analysis was performed with some of the variables used in the long-term model.
In the CURE study, the RR of suffering an event with clopidogrel compared to the control treatment was 0.80 (95% CI, 0.72-0.89). A sensitivity analysis was performed for the RR values between 0.70 and 0.90. Since no consensus was reached on whether the benefits to health should be discounted, or of what type of discount should be applied,26 the sensitivity analysis was performed without discounting the clinical benefits of clopidogrel. In addition, the use of resources can vary in normal clinical practice, and some authors indicate that this procedure usually underestimates true costs to some extent.27 To study the effect of the uncertainty of resource use on the robustness of the results, sensitivity analysis was performed varying by ±10% the number of patients using resources associated with each cost chapter (admissions, consultations, tests, and procedures).
Finally, the impact of patient age (50-75 years) and of varying the total health costs and pharmaceutical cost of clopidogrel by ±10% was investigated.
RESULTS
Effects on Health
For every 1000 patients with NSTEACS who received clopidogrel+STh during the first year of treatment (followed by STh) 21 cardiovascular events were avoided (cardiovascular death, AMI, or stroke) and 10 major hemorrhages produced (compared to those who received STh alone). When the results were analyzed by TIMI score risk group, clopidogrel+STh avoided 16 events per 1000 low risk (4.1% vs 5.7%) and intermediate risk patients (9.8 vs 11.4%), and 48 events per 1000 high risk patients (15.9% vs 20.7%).
In the long-term analysis, the model predicted a mean survival of 9.76 years for the patients treated with clopidogrel+STh and 9.65 years for those treated with STh alone. Therefore, providing clopidogrel during the first year of treatment led to an average 0.117 life-years gained per patient (117 for a cohort of 1000).
Effects on Resources
Tables 1 and 2 show the standard management patterns for AMI with and without Q wave, and the treatment for stroke, in the acute phase (i.e., which includes the first year) and in the second year and subsequent years. Table 3 shows the unit costs of the resources required. Table 4 shows the unit costs and percentage use of the drugs administered for the management of AMI and stroke in the acute phase and during the second/subsequent years. The cost of hemorrhage was obtained from that assigned to the Grupo Relacionado de Diagnóstico number 174 (gastrointestinal hemorrhage) (mean cost per patient, €2539.50).
The aggregate costs of each event and health state contemplated ascended to €7603.91 for AMI during the first year, €663.35 during the second and subsequent years following an AMI, €4957.38 for stroke during the first year, and €348.01 during the second and subsequent years following stroke.
Rests of the Cost-Effectiveness Analysis
Table 5 shows the results of the short-term analysis and those of the long-term model with respect to different time horizons. In the long-term analysis at 30 years, the incremental cost of treating with clopidogrel+STh compared to STh alone was €953 with an ICER of €8132 per LYG.
In the short-term analysis, the incremental cost per event avoided by administering clopidogrel+STh during the first year of treatment was €17,190. The incremental cost of clopidogrel+STh compared to STh alone was €361, of which €12 corresponded to the increase in major hemorrhages. If the ICER for the low, medium and high risk groups (according to TIMI score) is analyzed, assuming the same use of resources in each group, a result of €322,563 is obtained for every event avoided among low and medium risk patients, and €7520 for every event avoided among high risk patients.
Results of the Sensitivity Analysis
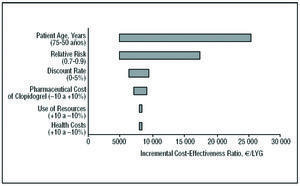
The results of the sensitivity analysis for the long-term model with respect to the RR of suffering an event, the use of resources, the discount rate, patient age, health costs, and the pharmaceutical cost of clopidogrel, were represented in a tornado diagram (Figure 2). This diagram shows the result of altering the values of the variables over the range considered.
Figure 2. Tornado diagram: sensitivity analysis and incremental cost-effectiveness of the use of clopidogrel plus standard therapy compared to standard therapy alone for the CURE population, in the long-term and modifying the variables: patient age, relative risk of events, benefit discount rate, the pharmaceutical cost of clopidogrel, use of resources associated with heath state, and health costs. LYG indicates life-years gained.
The variables with the greatest impact on the result were RR and patient age. If clopidogrel were to reduce the risk of suffering an event by 30% (RR=0.7) the ICER would increase to €5041 per LYG; if it reduced the risk by 10% (RR=0.9), the ICER would increase to €17 431 per LYG. Therefore, as the RR of suffering an event decreases with clopidogrel+STh (thus increasing the number of events avoided), its cost-effectiveness increases. The cost-effectiveness of treatment with clopidogrel+STh also increases with patient age. For a mean age of 50 years, the ICER is €25 509 per LYG. Adding clopidogrel to STh improved cost-effectiveness in scenarios in which the consumption of health resources was greater than that of the baseline scenario (the ICER was €8280 per LYG for the 10% scenario compared to €7968 per LYG for the scenario in which resource use was 10% above that of the baseline scenario). Figure 2 also shows the sensitivity analysis without applying the discount to the health costs and benefits (LYG). In this case, adding clopidogrel to STh was even more cost-effective than in the baseline scenario. Finally, varying the total health costs, use of resources and pharmaceutical costs of clopidogrel by ±10% had no significant effect on the results predicted by the model, the ICER being around that of the baseline value (Table 5).
DISCUSSION
The results of this study, which was adapted to the Spanish health setting, are consistent with economic assessments of clopidogrel use in other countries. In all cases the conclusion was reached that clopidogrel+STh during the first year of treatment is not only an effective but also a cost-effective treatment. In Denmark, Finland, Norway, Sweden, and the Netherlands,28,29 treatment with clopidogrel+STh was found to be cost-effective in the CURE study setting both in the short term and long term (determined via the projection of epidemiological data). The short-term cost-effectiveness of clopidogrel+STh compared to STh alone has also been studied in Belgium, Switzerland, Italy, the USA, Canada, France, and the UK, and the same conclusions reached.17,30
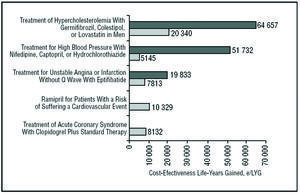
The results obtained in the present analysis are within the range in which the use of clopidogrel+STh can be considered cost-effective and therefore an efficient health intervention. In a recent review of the literature on economic assessment in Spain, the authors of the different evaluations recommended the adoption of interventions with an additional cost per LYG of <€30 000; for interventions with higher additional costs, no clear tendency in the recommendations was seen.31 In the present study, the cost effectiveness of the evaluated treatment was only €8132 per LYG, indicating that the National Health System should adopt clopidogrel+STh treatment. Some cost-effectiveness studies in Spain report a number of measures with less favorable cost-effectiveness figures to have been widely adopted by the National Health System (Figure 3).32-35
Figure 3. Incremental cost effectiveness ratio of different health interventions in Spain. LYG indicates life-years gained. aPlans-Rubio et al, 1995,32 bPlans-Ru-bio et al, 1998,33 cAntoñanzas et al, 2001,35 dHart et al, 2002.34 eResult of present study. The double bars refer to the maximum and minimum values of the cost-effectiveness ratio obtained in each study.
A recently published cost-utility analysis by Latour-Pérez et al36 was the first to analyze the long-term impact of clopidogrel in Spain.36 In some aspects the latter study differs substantially from the present work, for example in the length of the model cycles, the health states contemplated, and in the method for determining the costs. The most important difference, however, is that the former measures life years adjusted by quality of life. The utility data were obtained from different countries. Thus, the study of Latour-Pérez et al36 and the present study complement one another; the trend of the results is very similar although different methodologies were used and different measurements were made.
In the short-term analysis, the greater the risk faced by the patient the more efficient the use of clopidogrel became; the ICER fell from €22 563 in low risk (TIMI 0-2) and intermediate risk (TIMI 3-4) patients to €7521 in high risk (TIMI 5-7) patients.
The present study has a number of important limitations. Firstly, the data on the duration of the benefits of clopidogrel+STh treatment only refer to the first year, i.e., the time horizon for which direct empirical evidence is available. If the results of clinical trials currently underway were to show the benefit of clopidogrel+STh to go beyond one year, then an associated reduction in RR would substantially increase its cost-effectiveness. Secondly, the epidemiological data used to estimate the probability of transition between cycles were obtained using a model based on the Swedish population since no such information is available for its Spanish counterpart. However, both the CURE study population and the Swedish population (very similar to that of the CURE study; data gathered from the above-mentioned registries)19 are representative of the Spanish population with NSTEACS in terms of age, sex, and prior AMI. Thirdly, the Markov model used does not contemplate the possibility of transit between the health states pertaining to AMI and stroke. Therefore, the probability of suffering a stroke after an AMI or vice versa was not taken into account. According to Kannel,37 a patient with AMI has a 3-4-fold greater risk of suffering a stroke than members of the general population, and a patient who has suffered a stroke is at a 2-3 times greater risk of suffering an AMI. Another possible limitation is the fact that the study only took into account the direct costs of treatment and events. However, avoiding cardiovascular and cerebrovascular events and their associated mortality through the use of clopidogrel+STh during the first year of treatment provides benefits in terms of preventing losses of productivity through absenteeism, medically-ordered absence from work, and temporary or permanent incapacitation. None of these factors were taken into account in the present study. In any event, it would appear clear that the inclusion of this type of cost would favor the use of clopidogrel. The univariate sensitivity analysis showed that the variables that most influenced the results were the RR and mean age of the patient. Nonetheless, the cost per life-year when the percentage of events avoided through the use of clopidogrel was 10% or when patient mean age was 50 years was <€26 000; in other words, still within the efficiency range.
Bearing in mind the results obtained and the outcome of the sensitivity analysis, the present study can be considered sufficiently robust to allow the affirmation that administering clopidogrel+STh during the first year of treatment is cost effective.
CONCLUSIONS
In patients with NSTEACS, adding clopidogrel to STh with acetylsalicylic acid during the first year of treatment is cost effective from the viewpoint of the Spanish National Health System, both in the short and long term.
ABBREVIATIONS
LYG: life-years gained.
AMI: acute myocardial infarction.
ICER: incremental cost-effectiveness ratio.
RR: relative risk.
NSTEACS: acute coronary syndrome without ST segment elevation.
STh: standard therapy.
Correspondence: Dr. X. Badia.
Health Outcomes Research Europe.
Avda. Diagonal, 618, 1.o C-D. 08021 Barcelona. España.
E-mail: xbadia@hor-europe.com