The development of human-leukocyte antigen antibodies is a well-known adverse effect of the use of long-term ventricular assist devices (VADs). The aim of this study was to determine the incidence of sensitization during short-term mechanical circulatory support with VAD (CentriMag), its determinants, and its impact on posttransplant outcomes.
MethodsWe performed a retrospective review of patients who were bridged to transplant with short-term VAD from 2009 to 2019. Sensitization was defined as a calculated panel-reactive antibody> 10%. The endpoints included overall survival and rejection-free survival.
ResultsA total of 89 patients (median age 56.0 [interquartile range, 50.0-59.9] years, 16.8% female) received a short-term VAD as a bridge to transplant. The median duration of support was 23.6 [interquartile range, 16.6-35.0] days. Eleven patients (12.4%) became sensitized during support. The only factor significantly associated with sensitization was female sex (OR, 8.67; 95%CI, 1.93–38.8; P=.005). Of the 89 patients, 21 patients died during support; 68 patients underwent heart transplant. After a mean follow-up of 49.6 ±31.2 months, 8 patients (11.8%) died and 20 (29.4%) had at least 1 rejection episode. On multivariate analysis, sensitization was an independent predictor of acute rejection (HR, 3.64; 95%CI, 1.42-9.33; P=.007), with a nonstatistically significant trend to higher mortality (HR, 4.07; 95%CI, 0.96-17.3; P=.057).
ConclusionsSensitization with short-term VADs can occur and is significantly associated with female sex and with rejection. Sensitization also showed a nonstatistically significant trend to higher mortality.
Keywords
Medium- to-long-term ventricular assist devices (VADs) are known to mediate the production of anti-human-leukocyte antigen (HLA) antibodies. A range of factors have been linked to this phenomenon, including host immune responses to device biomaterials, multiple blood transfusions, female sex, multiparity, and previous surgery.1 Sensitization could have a negative impact on posttransplant outcomes, as it has been associated with a higher risk of graft vascular disease and acute rejection (AR), especially that mediated by antibodies.1,2 Some but not all studies have reported an increase in posttransplant mortality.2,3
Short-term VADs are not widely used as a direct bridge to transplant. Most often, they are used as a transition to longer-term circulatory support systems. Few studies have thus analyzed the incidence or prognostic implications of sensitization in the setting of short-term VAD support.4 Short-term devices, however, are the most used bridge-to-transplant system in Spain because of high access to transplantation and limited availability of long-term VADs due to budget constraints.5
The current literature on the impact of sensitization in patients on VAD support focuses on patients on long-term bridge-to-transplant systems.6 The aims of this study were to evaluate the incidence and determinants of sensitization in patients bridged to transplant with a short-term CentriMag VAD, describe the strategies used to treat sensitization, and investigate the impact of sensitization on posttransplant outcomes.
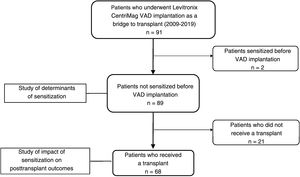
MethodsStudy design and populationWe performed a retrospective observational study of patients who received a short-term VAD (CentriMag, Abbott, United States) as a bridge to transplant at our hospital between July 2009 and November 2019 (figure 1). We recorded demographic and clinical characteristics (age, sex, underlying cardiomyopathy, diabetes mellitus, kidney failure, previous heart surgery), support characteristics (type of device [right, left, or biventricular], duration, reinterventions, pre-VAD extracorporeal membrane oxygenation, intra-aortic balloon contrapulsation, infections, and transfusions), and sensitization status. We also recorded details of induction and desensitization treatments and pretransplant immunosuppression for the subgroup of patients who received a heart transplant.
The respective primary and secondary endpoints were all-cause mortality and AR incidence during posttransplant follow-up. AR was defined as a clinical event— 2004 International Society of Heart and Lung Transplantation (ISHLT) grade ≥2R rejection diagnosed by endomyocardial biopsy or a >10% reduction in ejection fraction or worsening to <40%— requiring a transient increase in immunosuppression with a short course of oral or intravenous corticosteroids with or without cytolytic therapy.7
The study was approved by the pertinent clinical research ethics committee.
Short-term bridge-to-transplant VAD protocolShort-term VAD implantation is an established treatment for patients with end-stage heart failure in other countries in our setting. In Spain, however, because of the increased availability of donor hearts in recent years, short-term VADs are used as a bridge to transplant in patients who experience clinical deterioration while waiting for a heart transplant and patients with an advanced INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) level. Our hospital uses Levitronix devices for bridging as it considers they have greater potential to provide adequate support and optimal organ and functional recovery for subsequent transplant. Even patients with deep cardiogenic shock requiring percutaneous venoarterial extracorporeal membrane oxygenation are switched to a central VAD support system once the option of recovery has been ruled out and the patient is deemed eligible for transplant.
Patients are added to the waiting list following organ recovery and functional improvement. Invasive mechanical ventilation, unresolved infections, and persistent organ failure are generally considered contraindications for inclusion.
The VAD implantation procedure of choice is midline sternotomy with cannulation of the right atrium and pulmonary artery for right VADs and the left ventricular apex and the ascending aorta for left VADs.
Timing of anticoagulation initiation and establishment of treatment goals are tailored to individual hemorrhagic and thrombotic risk, but anticoagulants are never given until at least 16hours posttransplant. Sodium heparin is the anticoagulant of choice and anti-factor Xa and activated partial thromboplastin time monitoring is used for dose adjustments.
Antibody measurementsLuminex technology with LabScreen Single Antigen Class I and Class II reagents (One Lambda) was used to screen for anti-HLA antibodies in selected sera before elective VAD implantation. Additional measurements were performed 2 weeks after a sensitizing event (where feasible) and before inclusion on the heart transplant waiting list. Sensitizing events consisted of blood product transfusions and surgical reinterventions (including device replacement) during VAD support.
The anti-HLA specificities detected by Luminex were entered into the Eurotransplant calculator8 to obtain calculated panel-reactive antibody (cPRA) values before and after VAD implantation. For the purpose of this study, sensitization was defined as a cPRA value >10%.1,6
Statistical analysisContinuous variables are described as mean±SD for normally distributed data and median [interquartile range] for nonnormally distributed data. Normality of distribution was tested using the Kolmogorov-Smirnov test. Categorical variables are expressed as number and percentage. Differences between sensitized and nonsensitized patients were analyzed using the t test or Mann-Whitney U test for continuous variables and the chi-square test or Fisher exact test for categorical variables.
Predictors of sensitization were explored using logistic regression. Time to events (all-cause posttransplant mortality and AR) was analyzed by Cox regression. Two models were built for the multivariate analysis: model 1, which included variables with a significance <0.10 in the univariate analysis and model 2, which included variables with a significance <0.20. The variables were entered by stepwise backward elimination. All the tests were 2-tailed and significance was set at P<.05. The statistical analysis was performed in SPSS 25.0 (SPSS Inc, United States).
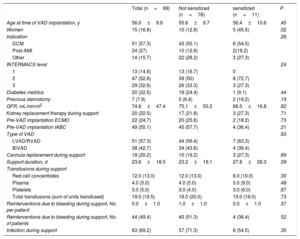
ResultsPatient characteristicsNinety-one patients received a short-term Levitronix CentriMag VAD as a bridge to transplant at our hospital between 2009 and 2019. Two patients with high cPRA values prior to implantation were excluded from the analysis (figure 1). The final sample thus comprised 89 patients (16.8% women) with a median age of 56 [50.0-59.9] years. The most common underlying heart condition was dilated cardiomyopathy (51 patients, 57.3%). Twenty-three patients (25.84%) were already on the waiting list for elective heart transplant and had undergone prior anti-HLA antibody screening. After VAD implantation, anti-HLA antibody levels were measured at least once before a patient was deemed eligible for priority transplant (code 0). Post–sensitizing-event measurements were feasible in 44.9% of patients. Patients underwent a mean of 1.55 ±0.5 measurements. The main type of VAD implanted was a left device (56.2% of patients), and the median duration of support was 23.6 [16.6-35.0] days. The characteristics of the 89 patients are shown by sensitization status in table 1.
Patients’ baseline characteristics
| Total (n=89) | Not sensitized (n=78) | sensitized (n=11) | P | |
|---|---|---|---|---|
| Age at time of VAD implantation, y | 56.0±9.9 | 55.6±9.7 | 56.4±10.6 | .45 |
| Women | 15 (16.8) | 10 (12.8) | 5 (45.5) | .02 |
| Indication | .26 | |||
| DCM | 51 (57.3) | 43 (55.1) | 6 (54.5) | |
| Post-AMI | 24 (27) | 10 (12.8) | 2(18.2) | |
| Other | 14 (15.7) | 22 (28.2) | 3 (27.3) | |
| INTERMACS level | .24 | |||
| 1 | 13 (14.6) | 13 (16.7) | 0 | |
| 2 | 47 (52.8) | 39 (50) | 8 (72.7) | |
| 3 | 29 (32.6) | 26 (33.3) | 3 (27.3) | |
| Diabetes mellitus | 20 (22.5) | 19 (24.4) | 1 (9.1) | .44 |
| Previous sternotomy | 7 (7.9) | 5 (6.4) | 2 (18.2) | .19 |
| GFR, mL/min/m2 | 74.6±47.4 | 75.1±50.2 | 68.0±16.8 | .82 |
| Kidney replacement therapy during support | 20 (22.5) | 17 (21.8) | 3 (27.3) | .71 |
| Pre-VAD implantation ECMO | 22 (24.7) | 20 (25.6) | 2 (18.2) | .73 |
| Pre-VAD implantation IABC | 49 (55.1) | 45 (57.7) | 4 (36.4) | .21 |
| Type of VAD | .83 | |||
| LVAD/RVAD | 51 (57.3) | 44 (56.4) | 7 (63.3) | |
| BiVAD | 38 (42.7) | 34 (43.6) | 4 (36.4) | |
| Cannula replacement during support | 18 (20.2) | 15 (19.2) | 3 (27.3) | .69 |
| Support duration, d | 23.6±18.5 | 23.2±19.1 | 27.8±28.3 | .09 |
| Transfusions during support | ||||
| Red cell concentrates | 12.0 (13.0) | 12.0 (13.0) | 8.0 (10.0) | .30 |
| Plasma | 4.0 (5.0) | 4.0 (5.0) | 5.0 (9.0) | .48 |
| Platelets | 3.0 (5.0) | 3.0 (4.0) | 3.0 (6.0) | .87 |
| Total transfusions (sum of units transfused) | 19.0 (19.5) | 18.5 (20.0) | 19.0 (18.0) | .73 |
| Reinterventions due to bleeding during support, No. per patient | 0.0±1.0 | 1.0±1.0 | 0.0±1.0 | .37 |
| Reinterventions due to bleeding during support, No. of patients | 44 (49.4) | 40 (51.3) | 4 (36.4) | .52 |
| Infection during support | 63 (69.2) | 57 (71.3) | 6 (54.5) | .30 |
AMI, acute myocardial infarction; biVAD, biventricular assist device; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; GFR, glomerular filtration rate; IABC, intra-aortic balloon counterpulsation; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; RVAD, right ventricular assist device; VAD, ventricular assist device.
Values are expressed as No. (%) or mean±standard deviation.
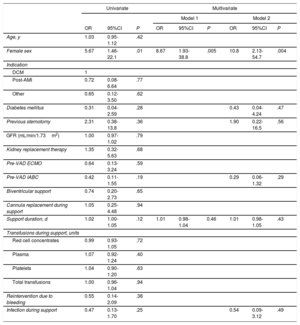
Eleven patients (12.4%) produced de novo anti-HLA antibodies while on VAD support, and they all received a heart transplant. The mean cPRA value was 45.6%±27.8% [11.0%-90.2%]. A mean fluorescence intensity cutoff of >1000 to 1500 was established to indicate positivity. Values of 500-1000 were classified as equivocal. Ten of the sensitized patients had a mean fluorescence intensity value >1500 (median, 5758 [3200-7000]). This information was not available for the other patient. All 11 patients had class I antibodies and 4 also had class II antibodies. There was a significantly higher proportion of women in the sensitized group than in the nonsensitized group (45.5% vs 12.8%, P=.02). We observed a nonsignificant trend toward an association between longer support duration and sensitization, with a median duration of 27.8 [23.6-51.8] days in the sensitized group vs 23.2 [15.2-34.3] days in the nonsensitized group (P=.09). In the univariate analysis, female sex was the only significant predictor of sensitization during VAD support (odds ratio [OR]=5.67; 95% confidence interval [95%CI], 1.46-22.1; P=.01) (table 2). Duration of support showed a trend toward significance (OR=1.02; 95%CI, 1.00-1.05; P=.12). The other variables showing a strong but nonsignificant trend toward an association with sensitization are listed in table 2. In the first multivariate model (model 1), which included sex and duration of support, only female sex retained its significance as a predictor of sensitization during support (OR=8.67; 95%CI, 1.93-38.8; P=.005). It was also the only independent predictor of sensitization in model 2 (OR=10.8; 95%CI, 2.13-54.7; P=.004) (table 2).
Determinants of sensitization during short-term circulatory support as a bridge to transplant
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Age, y | 1.03 | 0.95-1.12 | .42 | ||||||
| Female sex | 5.67 | 1.46-22.1 | .01 | 8.67 | 1.93-38.8 | .005 | 10.8 | 2.13-54.7 | .004 |
| Indication | |||||||||
| DCM | 1 | ||||||||
| Post-AMI | 0.72 | 0.08-6.64 | .77 | ||||||
| Other | 0.65 | 0.12-3.50 | .62 | ||||||
| Diabetes mellitus | 0.31 | 0.04-2.59 | .28 | 0.43 | 0.04-4.24 | .47 | |||
| Previous sternotomy | 2.31 | 0.38-13.8 | .36 | 1.90 | 0.22-16.5 | .56 | |||
| GFR (mL/min/1.73m2) | 1.00 | 0.97-1.02 | .79 | ||||||
| Kidney replacement therapy | 1.35 | 0.32-5.63 | .68 | ||||||
| Pre-VAD ECMO | 0.64 | 0.13-3.24 | .59 | ||||||
| Pre-VAD IABC | 0.42 | 0.11-1.55 | .19 | 0.29 | 0.06-1.32 | .29 | |||
| Biventricular support | 0.74 | 0.20-2.73 | .65 | ||||||
| Cannula replacement during support | 1.05 | 0.25-4.48 | .94 | ||||||
| Support duration, d | 1.02 | 1.00-1.05 | .12 | 1.01 | 0.98-1.04 | 0.46 | 1.01 | 0.98-1.05 | .43 |
| Transfusions during support, units | |||||||||
| Red cell concentrates | 0.99 | 0.93-1.05 | .72 | ||||||
| Plasma | 1.07 | 0.92-1.24 | .40 | ||||||
| Platelets | 1.04 | 0.90-1.20 | .63 | ||||||
| Total transfusions | 1.00 | 0.96-1.04 | .94 | ||||||
| Reintervention due to bleeding | 0.55 | 0.14-2.09 | .38 | ||||||
| Infection during support | 0.47 | 0.13-1.70 | .25 | 0.54 | 0.09-3.12 | .49 | |||
95%CI, 95% confidence interval; AMI, acute myocardial infarction; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; GFR, glomerular filtration rate; IABC, intra-aortic balloon counterpulsation; OR, odds ratio; VAD, ventricular assist device.
Model 1 included sex and support duration in days.
Model 2 included the variables from model 1 in addition to diabetes, previous sternotomy, pre-VAD IABC, and infection during support.
Twenty-one patients (23.6%) died during VAD support; the median time to death from implantation was 17.4 [3.3-23.2] days. The causes of death were infection (28.6%), ischemic stroke (14.3%), hemorrhage (14.3%), multiorgan failure (9.5%), medical futility (9.5%), hemorrhagic stroke (4.8%), and other (19%). Sixty-eight patients (76.4%) received a heart transplant after being supported for a median of 28.2 [19.6-39.8] days. Their demographic and clinical characteristics are summarized in the table 1 of the supplementary data.
All the patients were treated with an initial immunosuppression protocol consisting of tacrolimus, mycophenolate, and corticosteroids, with adjustments made as needed in patients at high risk for infection or kidney failure. Fifty-six (70%) of the nonsensitized patients received basiliximab induction therapy immediately after transplant. In the sensitized group, 10 patients (91%) were treated with thymoglobulin induction and 1 with basiliximab induction. Three patients with a posttransplant cPRA value >25% underwent desensitization with plasmapheresis and rituximab. This option was ruled out in another 3 patients with high sensitization levels (table 3), as it was considered that the risks outweighed the benefits. Retrospective crossmatching results were negative for 10 (91%) of the 11 patients in the sensitized group.
Characteristics, treatment strategies, and outcomes in patients who became sensitized during short-term bridge-to-transplant circulatory support
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 56 | 55 | 52 | 57 | 62 | 55 | 67 | 36 | 63 | 50 | 60 |
| Sex | Male | Male | Male | Male | Female | Female | Female | Male | Female | Male | Female |
| Indication for support | DCM | DCM | DCM | Post-AMI | DCM | HCM | Post-AMI | Congenital | Post-ECMO | DCM | DCM |
| Device | LVAD | LVAD | LVAD | BiVAD | BiVAD | LVAD | LVAD | LVAD | BiVAD | RVAD | LVAD |
| PRA, % | 30.0 | 11.0 | 19.7 | 22.0 | 23.4 | 79.0 | 74.0 | 39.6 | 90.2 | 70.0 | 43.0 |
| Anti-HLA class | I | I and II | I | I | I | I and II | I and II | I | I and II | I | I |
| Donor-specific antibodies | No | Possibly | No | No | No | No | No | No | No | No | No |
| Thymoglobulin induction | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Basiliximab induction | Yes | No | No | No | No | No | No | Yes | No | No | No |
| Plasmapheresis+rituximab | No | No | No | No | No | Yes | Yes | Yes | No | No | No |
| Posttransplant rejection | Yes | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Endomyocardial biopsy | No | No | - | - | - | - | Yes | Yes | No | No | No |
| Antibody-mediated rejection (ISHLT classification) | - | Yes/pAMR2 | - | - | - | - | Yes/pAMR2 | No | - | - | - |
| Cell-mediated rejection (ISHLT classification) | - | No | - | - | - | - | Yes/2R | Yes/2R | - | - | - |
| Posttransplant mortality | No | No | No | No | No | Yes | Yes | No | No | Yes | No |
| Death due to rejection | - | - | - | - | - | No | Yes | - | - | Yes | - |
AMI, acute myocardial infarction; BiVAD, biventricular assist device; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; HCM, hypertrophic cardiomyopathy; HLA, human-leukocyte antigen; ISHLT, International Society of Heart and Lung Transplantation; LVAD, left ventricular assist device; pAMR, pathological antibody-mediated rejection; PRA, panel-reactive antibody; RVAD, right ventricular assist device.
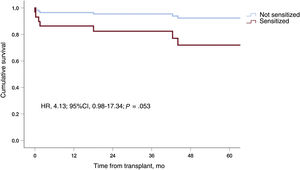
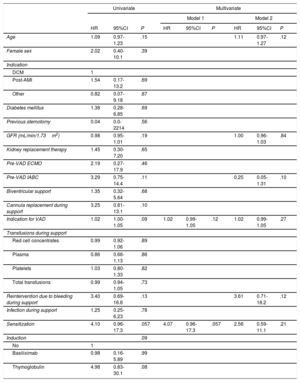
Eight (11.8%) of the 68 patients who received a heart transplant died after a median follow-up of 49.6±31.2 months: 3 (27.3%) were sensitized and 5 (8.8%) were not (P=.046) (hazard ratio [HR], 4.10; 95%CI, 0.96-17.28; P=.057). Pretransplant support duration showed a nonsignificant trend toward an increased risk of death (HR, 1.02; 95%CI, 1.00-1.05; P=.09) (table 4), as did induction therapy (P=.09) and VAD cannula replacement (P=.10). Induction therapy and cannula replacement were not included in the multivariate analyses because of potential collinearity. The first variable was strongly correlated with pretransplant sensitization (ρ=0.63; P=7.9×10–19), while the second was strongly correlated with support duration (ρ=0.76; P=6.3×10–14). In the abbreviated multivariate model (model 1), which only included sensitization status and support duration, pretransplant sensitization showed a trend toward an association with posttransplant mortality (HR, 4.07; 95%CI, 0.96-17.28; P=.057) (figure 2). The association in the more extensive second model was weaker and did not reach statistical significance (HR, 2.56; 95%CI, 0.59-11.1; P=.21) (table 4).
Determinants of posttransplant mortality in patients on short-term bridge-to-transplant circulatory support (Cox regression)
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Age | 1.09 | 0.97-1.23 | .15 | 1.11 | 0.97-1.27 | .12 | |||
| Female sex | 2.02 | 0.40-10.1 | .39 | ||||||
| Indication | |||||||||
| DCM | 1 | ||||||||
| Post-AMI | 1.54 | 0.17-13.2 | .69 | ||||||
| Other | 0.82 | 0.07-9.18 | .87 | ||||||
| Diabetes mellitus | 1.38 | 0.28-6.85 | .69 | ||||||
| Previous sternotomy | 0.04 | 0.0-2214 | .56 | ||||||
| GFR (mL/min/1.73m2) | 0.98 | 0.95-1.01 | .19 | 1.00 | 0.96-1.03 | .84 | |||
| Kidney replacement therapy | 1.45 | 0.30-7.20 | .65 | ||||||
| Pre-VAD ECMO | 2.19 | 0.27-17.9 | .46 | ||||||
| Pre-VAD IABC | 3.29 | 0.75-14.4 | .11 | 0.25 | 0.05-1.31 | .10 | |||
| Biventricular support | 1.35 | 0.32-5.64 | .68 | ||||||
| Cannula replacement during support | 3.25 | 0.81-13.1 | .10 | ||||||
| Indication for VAD | 1.02 | 1.00-1.05 | .09 | 1.02 | 0.99-1.05 | .12 | 1.02 | 0.99-1.05 | .27 |
| Transfusions during support | |||||||||
| Red cell concentrates | 0.99 | 0.92-1.06 | .89 | ||||||
| Plasma | 0.86 | 0.66-1.13 | .86 | ||||||
| Platelets | 1.03 | 0.80-1.33 | .82 | ||||||
| Total transfusions | 0.99 | 0.94-1.05 | .73 | ||||||
| Reintervention due to bleeding during support | 3.40 | 0.69-16.8 | .13 | 3.61 | 0.71-18.2 | .12 | |||
| Infection during support | 1.25 | 0.25-6.23 | .78 | ||||||
| Sensitization | 4.10 | 0.96-17.3 | .057 | 4.07 | 0.96-17.3 | .057 | 2.56 | 0.59-11.1 | .21 |
| Induction | .09 | ||||||||
| No | 1 | ||||||||
| Basiliximab | 0.98 | 0.16-5.89 | .99 | ||||||
| Thymoglobulin | 4.98 | 0.83-30.1 | .08 | ||||||
95%CI, 95% confidence interval; AMI, acute myocardial infarction; DCM, dilated cardiomyopathy; GFR, glomerular filtration rate; IABC, intra-aortic balloon counterpulsation; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; VAD, ventricular assist device.
Model 1 included sensitization status and duration of support.
Model 2 included the variables from model 1 in addition to age, glomerular filtration, reintervention during support, and pre-VAD IABCP.
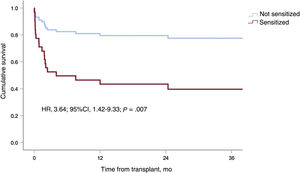
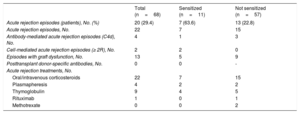
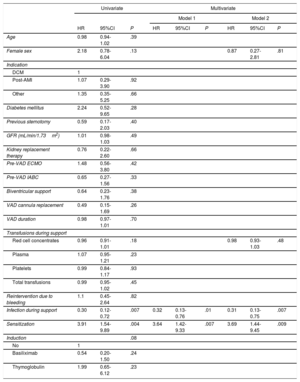
Twenty patients (29.4%) experienced at least 1 AR episode during follow-up (18 patients, 1 episode; 2 patients, 2 episodes). The median time from transplant to AR was 1.6 [0.1-63.9] months. As demonstrated by endomyocardial biopsy, 4 of the episodes were antibody-mediated and 2 were cell-mediated. In total, 59.1% of the episodes were classified as graft dysfunction (>10% reduction in ejection fraction or worsening to <40%). The main characteristics of AR are shown by sensitization status in table 5. Seven patients in the sensitized group (63.6%) experienced AR compared with 13 patients in the nonsensitized group (23.2%, P=.01). Sensitization was associated with a significantly higher risk of a first AR episode (HR, 3.90; 95%CI, 1.54-9.90; P=.004). Infection during VAD support exerted a protective effect against AR (HR, 0.30; 95%CI, 0.12-072; P=.007). None of the other variables were significantly associated with AR, although a trend was observed for pretransplant induction therapy (table 6). This variable was not included in the adjusted models because of its strong correlation with pretransplant sensitization status. Sensitization retained its significance as an independent predictor of AR (HR, 3.64; 95%CI, 1.42-9.33; P=.007) in both multivariate models (table 6, figure 3).
Characteristics of acute rejection and diagnostic-treatment protocol according to sensitization status
| Total (n=68) | Sensitized (n=11) | Not sensitized (n=57) | |
|---|---|---|---|
| Acute rejection episodes (patients), No. (%) | 20 (29.4) | 7 (63.6) | 13 (22.8) |
| Acute rejection episodes, No. | 22 | 7 | 15 |
| Antibody-mediated acute rejection episodes (C4d), No. | 4 | 1 | 3 |
| Cell-mediated acute rejection episodes (≥ 2R), No. | 2 | 2 | 0 |
| Episodes with graft dysfunction, No. | 13 | 5 | 9 |
| Posttransplant donor-specific antibodies, No. | 0 | 0 | - |
| Acute rejection treatments, No. | |||
| Oral/intravenous corticosteroids | 22 | 7 | 15 |
| Plasmapheresis | 4 | 2 | 2 |
| Thymoglobulin | 9 | 4 | 5 |
| Rituximab | 1 | 0 | 1 |
| Methotrexate | 0 | 0 | 2 |
Determinants of acute rejection in patients on short-term bridge-to-transplant circulatory support before transplantation (Cox regression)
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Age | 0.98 | 0.94-1.02 | .39 | ||||||
| Female sex | 2.18 | 0.78-6.04 | .13 | 0.87 | 0.27-2.81 | .81 | |||
| Indication | |||||||||
| DCM | 1 | ||||||||
| Post-AMI | 1.07 | 0.29-3.90 | .92 | ||||||
| Other | 1.35 | 0.35-5.25 | .66 | ||||||
| Diabetes mellitus | 2.24 | 0.52-9.65 | .28 | ||||||
| Previous sternotomy | 0.59 | 0.17-2.03 | .40 | ||||||
| GFR (mL/min/1.73m2) | 1.01 | 0.98-1.03 | .49 | ||||||
| Kidney replacement therapy | 0.76 | 0.22-2.60 | .66 | ||||||
| Pre-VAD ECMO | 1.48 | 0.56-3.80 | .42 | ||||||
| Pre-VAD IABC | 0.65 | 0.27-1.56 | .33 | ||||||
| Biventricular support | 0.64 | 0.23-1.76 | .38 | ||||||
| VAD cannula replacement | 0.49 | 0.15-1.69 | .26 | ||||||
| VAD duration | 0.98 | 0.97-1.01 | .70 | ||||||
| Transfusions during support | |||||||||
| Red cell concentrates | 0.96 | 0.91-1.01 | .18 | 0.98 | 0.93-1.03 | .48 | |||
| Plasma | 1.07 | 0.95-1.21 | .23 | ||||||
| Platelets | 0.99 | 0.84-1.17 | .93 | ||||||
| Total transfusions | 0.99 | 0.95-1.02 | .45 | ||||||
| Reintervention due to bleeding | 1.1 | 0.45-2.64 | .82 | ||||||
| Infection during support | 0.30 | 0.12-0.72 | .007 | 0.32 | 0.13-0.76 | .01 | 0.31 | 0.13-0.75 | .007 |
| Sensitization | 3.91 | 1.54-9.89 | .004 | 3.64 | 1.42-9.33 | .007 | 3.69 | 1.44-9.45 | .009 |
| Induction | .08 | ||||||||
| No | 1 | ||||||||
| Basiliximab | 0.54 | 0.20-1.50 | .24 | ||||||
| Thymoglobulin | 1.99 | 0.65-6.12 | .23 | ||||||
95%CI, 95% confidence interval; AMI, acute myocardial infarction; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; GFR, glomerular filtration rate; HR, hazard ratio; IABC, intra-aortic balloon counterpulsation; VAD; ventricular assist device.
Model 1 included sensitization status and infection during support.
Model 2 included the variables from model 1 in addition to sex, diabetes, ventricular assist device cannula replacement, and sum of blood products transfused.
Induction therapy was not included in the multivariate models due to its strong correlation with sensitization.
This study has shown that sensitization can occur in patients on short-term VAD support as a bridge to transplant. The incidence in our series was around 12% and was significantly higher in women. Sensitization affected posttransplant outcomes, mainly in the form of an increased incidence of AR.
Sensitization during long-term VAD support has been studied extensively, and incidence rates vary widely depending on the definition of sensitization and the type of study.9 Sensitization poses a clinical challenge in bridge-to-transplant settings due to the complications caused by the production of antibodies and the risk of adverse effects during desensitization treatment (infection, kidney failure, coagulopathy, lymphoproliferative disease).10 The cPRA cutoff of 10% for defining sensitization has been used in previous studies; patients are considered to be highly sensitized at values ranging from >50% to 90% depending on the study.1,6
The increased incidence of sensitization during long-term VAD support appears to be linked to an increase in the host's immunologic and inflammatory response in relation to host-device interactions and multiple transfusions.11 It has been postulated that type of device might influence the incidence of sensitization, with higher rates affecting patients with pulsatile-flow VADs due to the constant stimulus of lymphocytes (these devices require biological valves and a greater contact surface with the blood).11 Other factors linked to sensitization in this setting are young age and a history of pregnancy, blood product transfusion, pre-VAD sensitization, and heart surgery.6 In our study, where all the patients were treated with the same type of short-term VAD (CentriMag), female sex was the only independent predictor of sensitization during support, suggesting a possible link with a history of pregnancy-related alloimmunization. We also observed a higher but nonsignificant incidence of anti-HLA antibodies in patients who had been on support for longer; this association might have reached statistical significance if the sample had been larger. Even though blood product transfusion is generally considered to be a sensitizing event, it was not an independent predictor of sensitization in our series, possibly because our hospital generally uses leukodepleted blood products. No associations were observed either for age, previous heart surgery, or surgical reinterventions.
The production of anti-HLA antibodies, detected by screening (in addition to crossmatching), has been linked to worse posttransplant outcomes in the form of AR, graft vascular disease, and death. In a study of 8160 sensitized heart transplant recipients, Nwakanma et al.2 showed that a PRA value >25% was significantly associated with shorter survival times and higher rejection rates. Most studies, however, have not identified a link between sensitization during VAD support and worse posttransplant outcomes.1,7 Alba et al.,1 for example, found no effect on rejection or mortality rates, possibly because they used more aggressive immunosuppression protocols and closer rejection risk monitoring. Sensitization was also associated with a lower likelihood of receiving a transplant and longer waiting times. Unlike Alba et al., we did not perform virtual crossmatching. It should also be noted that the study by Alba et al. included patients on long-term VAD support selected for nonurgent heart transplant; there was therefore no pressing need to find a histocompatible donor. Our situation was completely different, as all the patients on the heart transplant waiting list were high-priority candidates supported by a VAD with a claimed duration of just 30 days. In addition, donor HLA typing results are not always available in our setting because of the need to limit ischemic times during transplant.
The 3 sensitized patients who died after a heart transplant in our series were all highly sensitized. Despite the limiting factors described above, prospective virtual crossmatching would probably have improved their prognosis by enabling us to select HLA-compatible donors.
This study, to our knowledge, is the first to analyze the incidence and prognostic implications of sensitization in a cohort of patients on short-term circulatory support with a CentriMag-type VAD as a bridge to transplant. We found that patients who became sensitized were significantly more likely to experience a first AR episode. The trend observed for the association between sensitization and mortality was not significant but it might be clinically relevant. Infection exerted a protective effect against AR. A higher incidence of infection is a recognized marker of diminished immunity. Tests such as ImmuKnow could also help identify patients at greater risk of infection or AR. They are not, however, routinely performed, and are not available at our laboratory.
Anti-HLA antibodies in our series were measured by Luminex before elective VAD implantation, 2 weeks after a sensitizing event (if the patient had not already received a transplant), and again before transplant. Ten of the 11 sensitized patients underwent desensitization with thymoglobulin induction therapy in the immediate posttransplant period. In addition, the 3 patients with a cPRA >25% (27.3%) were treated with pretransplant plasmapheresis and rituximab. Desensitization was ruled out in the other 3 with high cPRA values, as the risks were considered to outweigh the potential benefits.
The treatment of sensitization in patients on VAD support is not standardized and an optimal desensitization strategy has yet to be defined.6,10 Most of the current knowledge is based on findings from small observational studies.12 Solid-phase anti-HLA antibody testing (Luminex) is the gold standard because of its superior sensitivity, specificity, and reproducibility. Additional tests, such as the complement fixation test, can be useful for determining the clinical relevance of sensitization, as complement-fixing ability has been linked to early AR.13 This technique, however, has not yet been standardized by most laboratories. The specific nature of antibodies produced can also affect the prognostic impact of sensitization. In our study, 91% of patients had negative crossmatch results, possibly indicating that the antibodies detected were mostly non–donor-specific or that they had been cleared by desensitization treatment. Nonetheless, an increased risk of AR has been observed in sensitized patients without donor-specific antibodies.2 Previous studies have indicated that antibodies directed against non-HLA antigens may have a role in antibody-mediated AR, and this possibility is mentioned in the ISHLT consensus document on the management of antibodies in heart transplantation, although more studies are needed to determine its clinical relevance.14,15
In line with common practice,2,10 we performed desensitization in patients with a cPRA value >25%-50%. The American Heart Association, in its algorithm on desensitization therapy, advises against desensitization and antibody monitoring in patients with cPRA values <20%. By contrast, the Association recommends monitoring for antibodies and rejection at a value of 20%-50% and considering the option of desensitization therapy at a value of >50%. It does not, however, make any specific recommendations for patients on short-term bridge-to-transplant VAD support.6
Hypersensitized patients are good candidates for desensitization treatment, which has 2 primary purposes: to remove circulating antibodies through extracorporeal cleaning techniques such as plasmapheresis and immunoadsorption and to reduce the production of de novo antibodies by B cells through drugs such as rituximab. This combined strategy is effective at reducing antibody titers and might also improve posttransplant outcomes.10 Choice of specific strategy largely depends on the transplant centre and individual risk-benefit ratios. It is important to remember that desensitization is associated with a particularly high risk of hemorrhagic and thrombotic complications and an increased risk of infection. These risks will influence the cPRA threshold and choice of strategy.
LimitationsBecause this was a retrospective observational study, our findings may have been affected by biases inherent to studies of this nature. The small number of patients and events will also have resulted in underpowering, which may have particularly affected our analysis of posttransplant mortality.
It would seem reasonable to assume that the risk of sensitization increases with support duration. This relationship, however, might have a spurious component, as patients awaiting the results of desensitization therapy might have to wait longer for a transplant.
At our hospital, we monitor risk of rejection through periodic clinical and echocardiographic evaluations, and only perform biopsy in equivocal cases or following suspicious clinical events. The lack of histopathologic findings to support a diagnosis of AR is another limitation of our study.
Sensitization levels (cPRA values) may also vary, as they depend on the composition of the antibody panel and the detection method used.
Nonetheless, our study describes clinical practice in a group of heart transplant patients over a period of 10 years and is the first to focus on treatment strategies and outcomes in patients who become sensitized during short-term VAD support.
ConclusionsThe incidence of sensitization in patients on short-term VAD support is around 12% and is particularly high in women. Sensitization can affect posttransplant outcomes, with our findings showing a significant association with an increased risk of AR and a nonsignificant trend toward higher mortality.
- –
Long-term VAD support is associated with an increased production of anti-HLA antibodies due to device immunogenicity and the frequent need for multiple transfusions in this setting. Both these factors are well-established risk factors for sensitization. Sensitization is associated with worse posttransplant outcomes in the form of AR, graft vascular disease, and death.
- –
No studies have analyzed the incidence or prognostic implications of sensitization during short-term VAD support.
- –
This is the first study to analyze sensitization during short-term VAD support.
- –
Sensitization can occur in this setting and is considerably more likely in women and significantly associated with an increased risk of AR.
- –
We have described strategies to diagnose and treat sensitization during short-term VAD support in a group of heart transplant patients.
None declared.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.01.017