Initial preclinical studies are required during the process of improving polymers, platforms, and drug-eluting systems for new coronary stent designs. Our objective was to analyze the efficacy and safety of new drug-eluting stent models compared with a conventional stent and commercialized drug-eluting stents in an experimental model with healthy porcine coronary arteries.
MethodsSixty stents (conventional stent, new sirolimus-eluting stents: drug-eluting stents 1, 2 and 3; Cypher® and Xience®) were randomly placed in the coronary arteries of 20 Large White domestic pigs. Angiographic and histomorphometric studies were done 28 days later.
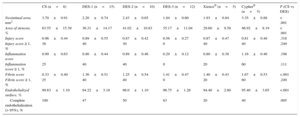
ResultsThe stents were implanted at a stent/artery ratio of 1.34±0.15, with no significant differences between groups. The new stents showed less late loss and angiographic restenosis than conventional stents (P=.006 and P<.001, respectively). Histologically, restenosis and neointimal area were lower with all the new platforms than with the conventional stents (P<.001 for each variable), and no differences were found vs the drug-eluting stents on the market. Safety data showed that endothelialization was lower with drug-eluting stents than with conventional stents, except for drug-eluting stent 3 (P=.084). Likewise, inflammation was lower with drug-eluting stent 3 than with other stents.
ConclusionsThe new drug-eluting stent platforms studied are associated with less restenosis than conventional stents and showed no significant differences in safety or efficacy vs commercialized drug-eluting stents.
Keywords
Drug-eluting stents (DES) are one of the greatest advances made in the percutaneous treatment of coronary disease. These devices have consistently provided lower rates of target vessel revascularization than conventional stents (CS) in a wide range of clinical situations.1–4 However, the risk of late and very late thrombosis associated with these stents is still a cause for concern.5,6 This phenomenon has been related to the deleterious effects of the drug, polymer, stent platform, or a combination of all 3 on the vessel wall, leading to incomplete endothelialization, persistent inflammatory reactions, and the development of neoatherosclerosis.7–11
To overcome these limitations, innovations have been made in platform design and drug-eluting systems, polymers have been developed that are more biocompatible or resorbable, and even a completely resorbable DES has been designed.12–14 Preclinical animal studies have been deemed very useful for analyzing the differences among new devices because the sequence of biological events associated with arterial healing (injury caused by the stent, fibrin deposits, inflammation and cell proliferation) is similar to that in humans.15–17 Experimental models with healthy porcine coronary arteries are considered suitable for evaluating biological responses after the placement of CS, DES or drug-eluting balloons.18–20
The purpose of this study was to evaluate the safety and efficacy of 3 new sirolimus-eluting permanent polymer stent designs in terms of vascular response in a preclinical porcine model.
METHODSAnimal ModelIn this randomized, controlled, experimental study with a final blind analysis, we used 20 Large White domestic pigs aged 2 to 3 months old and weighing 25±3kg. All procedures were done in accordance with local regulations (RD 53/2013, February 1, which defines basic standards for the protection of animals during experimentation and other scientific purposes, such as education) and European Directive 2010/63/EC. Before any procedures were initiated, the study was approved by the local ethics committee.
The randomization method involved the stratified allocation of major coronary arteries in such a way that each stent type was implanted in the same number of arteries.
All animals received antiplatelet therapy with acetylsalicylic acid (325mg) and clopidogrel (300mg) 24hours before the procedure. The anesthesia protocol and surgical preparation have been previously described.21,22 The animals were anesthetized and received anticoagulant therapy with 5000 IU of unfractionated heparin. Coronary angiography was performed via left carotid artery access after intracoronary administration of nitroglycerin.
Angioplasty ProcedureTo implant the devices and obtain a stent/artery ratio > 1.1, we selected the best location out of the 3 epicardial coronary arteries. After inserting an intracoronary guidewire, the different stent types were implanted in the selected area of each artery.
Devices AnalyzedFor this study, we used the following devices (numbers in parentheses):
- 1.
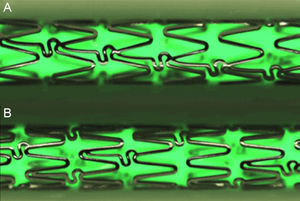
Control CS (n=11): L605 cobalt chromium alloy stent, Architect® (iVascular). The stent is constructed of 6 crowns joined by 3 rows of concatenated connectors that create a continuous sinusoidal structure (Figure 1A).
Figure 1.Stent design of the conventional cobalt chromium Architect®, the metallic control platform, drug-eluting stent 1 and drug-eluting stent 2 (A); the new metallic structural platform of the drug-eluting stent 3 has more crowns per segment and unlinked connectors to provide more uniform elution (B). High definition images using the QSix® system (Barcelona, Spain).
(0.12MB). - 2.
DES 1 (n=17): based on the metal Architect® stent, coated with a permanent polyacrylate polymer and loaded with 1.4μg/mm2 of sirolimus in a slow-release system (with an additional polymeric external layer to control drug release).
- 3.
DES 2 (n=10): based on the metal Architect® stent, coated with a permanent polyacrylate polymer and loaded with 1.4μg/mm2 of sirolimus (and no external polymer barrier).
- 4.
DES 3 (n=12): is the new DES by iVascular, called Angiolite®. The platform is made of L605 cobalt chromium alloy, with a strut thickness of 85μm. The stent structure has 8 crowns joined by 3 rows of unlinked connectors that create a discontinuous sinusoidal structure (Figure 1B). This design represents a slight increase in the metal/artery ratio and provides better distribution of the drug to the artery wall. The polymer is permanent and from the polyacrylate family. It releases sirolimus at a dose of 1.4μg/mm,2 and more than 80% of the drug is released within 60 days.
- 5.
DES 4 (n=5): commercial first-generation Cypher® DES (J&J Cordis; Miami Lakes, Florida, Unites States) with a strut thickness of 140μm, loaded with 1.4μg/mm2 of sirolimus, and permanent copolymer matrix.
- 6.
DES 5 (n=5): second-generation Xience® DES (Abbott Vascular; Santa Clara, California, United States). This stent elutes everolimus at a dose of 1μg/mm2 through its permanent fluorinated copolymer. The metallic platform is a Multi-Link stent, composed of a cobalt chromium alloy, with a strut thickness of 81μm.
All the material was provided by iVascular, including DES 1, DES 2 and DES 3, which are still not commercially available.
The sample size and number of stents included in the study were calculated according to consensus documents on preclinical stent analysis.20
Angiographic StudiesAfter the above-mentioned procedures were completed in each artery, coronary catheterization was repeated (after intracoronary administration of nitroglycerin) to determine the minimal luminal diameter (MLD) inside each stent. Catheterization was repeated 28 days later to evaluate follow-up MLD. Both MLDs, after the procedure and during follow-up, as well as the reference diameters of the arteries (mean diameter of the arterial segments located in the proximal and distal 5mm of the stent), were calculated with Medis QCA-CMS (version 6.1) software for quantitative coronary analysis. The following angiographic restenosis parameters were calculated:
- •
Late loss=initial MLD – follow-up MLD.
- •
Percentage of angiographic restenosis=[1-(initial MLD/reference diameter)]×100.
The presence of overlapping branches that impeded correct vessel measurement was considered a criterion for exclusion from the angiographic analysis.
Histologic AnalysisAfter completing the angiographic follow-up, the animals were killed and underwent complete histologic analysis. The hearts were explanted and coronary arteries were preserved by means of pressure perfusion, which was done initially with phosphate buffered saline and later using 4% paraformaldehyde. The treated segments were dissected, and the 5mm distal and proximal to the treated area were preserved. The samples were embedded in plastic resins to obtain circumferential sections representative of the proximal, medial, and distal areas and to calculate the mean values of each segment studied. Afterward, the sections were systematically stained with hematoxylin-eosin and Van Gieson stain.
The arteries were analyzed histomorphometrically with an Olympus PRovis AX70W digital microscope (Tokyo, Japan) paired with a Nikon DXM 1200W digital camera and ImageJ-NIH Image 1.4 software (National Institutes of Health, United States). Planimetry was used to determine the luminal area and the internal elastic lamina area and thus calculate restenosis variables defined by histology:
- •
Neointimal area=area of internal elastic – luminal area.
- •
Percentage of stenosis by histology=[1 – (luminal area / internal elastic area)]×100.
The histologic analysis in terms of safety was based on semiquantitative analysis of 4 parameters: degree of vascular injury (injury score), defined by Schwartz et al23; inflammation intensity, defined by Kornowski et al24; persistent fibrin deposition, according to Suzuki et al,25 and the degree of re-endothelialization, calculated as the approximate percentage of luminal surface covered by endothelial cells. According to the amount of surface covered by endothelial cells 28 days after implantation, an additional parameter was defined: complete endothelialization, which was at least 95% of the luminal surface covered by the endothelial cells.26 Stents with an injury score > 2 were excluded from the final analysis as they can have nonspecific responses in the histology of the arterial wall.
Statistical MethodValues are presented as proportions and as mean±standard deviation, depending on the type of variable. The semiquantitative variables, like the safety scores in the histopathologic analysis, are described as mean±standard deviation (the most common form in previous publications) and as percentages (recommended by consensus documents for preclinical stent studies).20,27
We analyzed the differences between the mean of the groups using Student's t test and analysis of variance. For multiple comparisons, a post-hoc analysis was done with the Dunnett method for comparison with the control CS and with the Tukey method for comparison of all groups. The semiquantitative variables were analyzed with the chi-square test or Fisher's exact method. To evaluate the possible influence of different variables (stent/artery ratio, treated artery, stent type and injury score) on the final results of angiographic and histologic stenosis, we carried out a multivariate linear regression analysis that included, in addition to the cited variables, the stent type (CS or DES). The variables were entered into the model as a block with a P value for entry of .05 and an output P of .1. For the analyses, a P<.05 was considered significant.
RESULTSIn this study, we finally implanted 59 of the 60 planned stents in porcine coronary arteries (1 CS could not be correctly implanted in the right coronary artery) and overdilated to a mean stent/artery ratio of 1.34±0.15, with no significant differences between stents. The animals completed the planned follow-up; angiographic follow-up studies and histologic analyses were done without incident. All the treated segments were permeable in the final angiographic analysis. Two CS, 2 DES-1 and 1 DES-3 were excluded from the final analysis because they had an injury score > 2. The angiographic analysis of 2 stents could not be completed due to the presence of overlapping branches in the stent segment of 1 case (DES-1 in circumflex artery) and inadequate opacification of the stent in the other (DES-3 in right coronary).
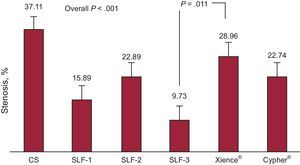
Analysis of Antirestenotic EffectivenessAntirestenotic properties are shown in Tables 1 and 2. In general, late loss was significantly lower for all the DES than for the control CS (P=.006). Late loss was lower for DES-1 and DES-3 than for the CS (P=.025 and P=.004, respectively). Likewise, values for this variable were lower for DES-3 than for the Xience® stent, a finding that was borderline statistically significant (P=.049). Angiographic restenosis was also lower in the DES group than in the control CS (P<.001); nevertheless, this was only true with the new prototypes tested (P<.001 for DES-1; P=.047 for DES-2; P<.001 for DES-3). Restenosis was lower for DES-3 than for the Xience® stent (P=.011) (Figure 2).
Angiographic Results at 28 Days
| CS (n=8) | DES-1 (n=14) | DES-2 (n=10) | DES-3 (n=11) | Xience® (n=5) | Cypher® (n=5) | P (CS vs DES) | |
|---|---|---|---|---|---|---|---|
| Late loss, mm | 1.54±0.40 | 1.03±0.55 | 1.26±0.30 | 0.87±0.30 | 1.52±0.37 | 1.19±0.30 | .006 |
| Stenosis, % | 37.11±12.73 | 15.89±10.59 | 22.89±7.92 | 9.73±6.09 | 28.96±9.86 | 22.74±14.14 | < .001 |
CS, conventional stent; DES, drug-eluting stent.
Data are shown as mean±standard deviation.
Histology Results at 28 Days
| CS (n=8) | DES-1 (n=15) | DES-2 (n=10) | DES-3 (n=12) | Xience® (n=5) | Cypher® (n=5) | P (CS vs DES) | |
|---|---|---|---|---|---|---|---|
| Neointimal area, mm2 | 3.70±0.91 | 2.20±0.74 | 2.43±0.65 | 1.84±0.60 | 1.93±0.84 | 3.35±0.88 | < .001 |
| Area of stenosis, % | 63.55±15.39 | 36.31±14.17 | 41.02±10.83 | 35.17±11.04 | 29.60±9.58 | 46.92±8.19 | < .001 |
| Injury score | 0.96±0.44 | 0.89±0.55 | 0.87±0.42 | 0.56±0.27 | 0.87±0.47 | 0.81±0.48 | .318 |
| Injury score ≥ 1, % | 38 | 40 | 30 | 0 | 40 | 40 | .249 |
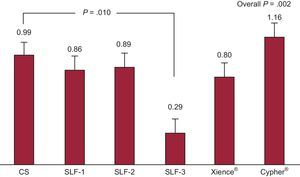
| Inflammation score | 0.99±0.63 | 0.86±0.44 | 0.89±0.46 | 0.29±0.12 | 0.80±0.38 | 1.16±0.46 | .198 |
| Inflammation score ≥ 1, % | 25 | 40 | 40 | 0 | 20 | 60 | .111 |
| Fibrin score | 0.33±0.40 | 1.36±0.51 | 1.25±0.54 | 1.41±0.47 | 1.40±0.43 | 1.67±0.53 | <.001 |
| Fibrin score ≥ 1, % | 25 | 40 | 40 | 0 | 20 | 60 | .249 |
| Endothelialized surface, % | 99.63±1.10 | 94.22±3.18 | 96.0±1.10 | 96.75±1.26 | 94.40±2.60 | 95.40±3.65 | <.001 |
| Complete endothelialization (> 95%), % | 100 | 47 | 50 | 83 | 20 | 40 | .005 |
CS, conventional stent; DES, drug-eluting stent.
Unless otherwise indicated, the data are shown as mean±standard deviation.
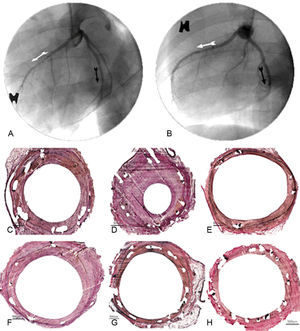
The histologic analysis showed that neointimal area and histologic restenosis were lower with the new sirolimus DES than with the CS (P<.001 for both variables). Neointimal area was significantly lower with the new devices than with the CS and the Cypher®, but no differences were found vs the Xience® stent. Likewise, histologic restenosis was lower with the new devices than with the CS, but was higher than with Xience® or Cypher®. The effects of the different stents, both in the angiography and in the histology studies are shown in Figure 3.
Antiproliferative effectiveness: angiographic and histologic comparison at 28 days. Angiographic results of conventional control stent (A: anterior descending, white arrow), drug-eluting stent 3 (A: circumflex, black arrow), drug-eluting stent 2 (B: anterior descending, white arrow) and drug-eluting stent 1 (B: circumflex, black arrow). Histology results of the Cypher® stent (C), conventional stent (D), Xience® (E), drug-eluting stent 1 (F), drug-eluting stent 2 (G) and drug-eluting stent 3 (H).
A multivariate analysis evaluated the influence of different variables on histologic restenosis. An independent association was found between a greater degree of restenosis and a higher injury score (B=13.38; 95% confidence interval, 5.68-21.08; P<.001) and lower levels in the case of DES vs control (B=–26.73; 95% confidence interval, –35.95 to 17.51; P<.001).
Safety AnalysisSafety variables are shown in Table 2. In general, at 28 days, endothelialization was greater with the CS than with the analyzed DES (P<.001). This difference was not statistically significant in the case of DES-3 (P=.084). No differences were observed among the DES analyzed. The injury score showed no significant differences between the devices. The degree of inflammation was even lower for DES-3 than for the CS (P=.010) (Figure 4). Lastly, fibrin deposits were lower in the CS group than in the tested DES (P<.001).
DISCUSSIONThis study analyzed the safety and efficacy of new sirolimus-eluting stent models with a permanent polymer compared with CS and 2 currently available DES. Our preclinical model showed that the new devices reduce the degree of restenosis (fundamentally DES-1 and DES-3) vs the control CS, with similar safety parameters as currently available DES.
Preclinical animal models are a fundamental part of the approval process and are essential for verifying the safety and efficacy of new coronary devices. Likewise, these models are the most effective method to evaluate mural response to new therapeutic materials.
The new DES models generally demonstrated greater efficacy in the prevention of restenosis than the control CS. This finding was expected, as previous studies had reported that different sirolimus-eluting stents significantly reduce late loss and target vessel revascularization compared with CS.1,2,25,28–30 Furthermore, in our study, DES-3 seemed to be the most effective stents in general, even when compared with commercially-available DES. This finding is relevant because recent meta-analyses have reported that the Xience® stent is the device with the best efficacy/safety ratio.31,32 Although this finding may be explained by various factors, previous publications indicate that neointimal hyperplasia and thrombus deposition can be significantly affected by stent design and coating.33–36 It is likely that these effects may be caused by the new design of this platform, with improved distribution of the drug along the artery wall. Despite these promising results, we must assume that the porcine coronary model has limitations for assessing mural response to stents, and that no animal model completely and precisely reproduces the characteristics of vascular disease in humans.20,27 This positive finding should therefore be interpreted with caution. Nonetheless, experimental studies indicate that there are important similarities between the inflammation, vascular injury and neointimal growth of these models and native human coronary arteries, although the vascular injury in animal models differs from that of atherosclerotic human arteries.37,38 Vascular injury is the fundamental event that could lead to the differences found among the stents studied in the porcine model. Our group has previously reported that a greater degree of vascular injury caused by stents overdilatation is a powerful stimulus for neointimal proliferation. Therefore, the greater the vascular damage, the greater the efficacy of the DES in preventing neointimal response.22 This phenomenon was found in our study, in which the stent/artery ratio was higher than recommended,20 which allowed differences to be observed among the devices. Furthermore, it is relevant that this overdilatation was not associated with an excessive injury score, which could have altered the results of the study by inducing nonspecific vascular responses. This would be confirmed by the absence of a significant association between this variable and the degree of angiographic or histologic stenosis. In our multivariate analysis, the use of DES was independently associated with a lower degree of stenosis, confirming that the effect is real and is not related to arterial injury.
The safety data showed that the degree of endothelialization was lower in the DES than in the control CS, which can be explained by the effect of sirolimus, a powerful inhibitor of endothelial proliferation that deactivates the p70 S6 kinase pathway, an essential step for the progression of the cell cycle in response to growth factors.28,39,40 Interestingly, DES-3 showed no significant differences compared with CS, confirming the safety of this device. Likewise, fibrin deposition around the stents was greater in the DES group, which is an indication of the effect of the drug.41 Importantly, injury scores were low for all the stents, with a mean < 1 despite a stent/artery ratio ≥ 1.3. This finding is probably a consequence of the high degree of biocompatibility of the tested material. Lastly, inflammatory response was lower with DES-3 than with the control CS. Although this finding could be explained by several factors, it is possible that this new design, with its improved sirolimus-eluting pattern, may be associated with a greater anti-inflammatory effect of the drug,42 which could counterbalance the proinflammatory effect of the permanent polymer.43
LimitationsSimilar to all preclinical models, this study has inherent limitations because no animal model can exactly reproduce the complex characteristics of human coronary disease. Animal models of disease can replicate some of them, but their exact interpretation is not quite clear. Although the proliferative response obtained is able to evaluate drug activity and stent behavior, it is unknown whether this effect would be the same in arteries with a large atherosclerotic content, in which fragmentation of the internal elastic lamina spontaneously appears as part of the general inflammatory process, especially in vulnerable plaques, in which other mediators participate in the vascular healing process. Furthermore, patients with atherosclerosis have other molecules and genetic factors that can directly interfere in the process. Nevertheless, the porcine coronary artery model continues to be recommended by consensus documents for the evaluation of these devices. Accuracy is also limited by histologic evaluation using semiquantitative variables; nonetheless, we have followed the standards for analysis postulated by expert consensus.20,27 The sample size was calculated in accordance with the standards defined by consensus documents for preclinical studies of coronary stents. Even though the power for detecting differences with CS is adequate, the power to detect differences among DES types is lower. These comparisons should therefore be interpreted with caution.
CONCLUSIONSIn our experimental model with healthy porcine coronary arteries implanted with oversized stents, the new sirolimus stents tested significantly reduced restenosis compared with the control CS. In addition, we found no relevant differences with the first- and second-generation DES currently available on the market.
FUNDINGThis study was completed with the support of the CDTI (Center for Industrial Technological Development) of the Spanish Ministry of Economics and Competitiveness (IDI-20111025).
CONFLICTS OF INTERESTA. Pérez de Prado and F. Fernández-Vázquez are consultants and receive funding for different research projects from LVD Biotech/iVascular. M. Molina and L. Duocastella are employed by LVD Biotech/iVascular. L. Duocastella is the CEO (Chief Executive Officer) and holds shares in LVD Biotech/iVascular.