Paclitaxel-eluting balloons have shown high antiproliferative efficacy in the treatment and prevention of restenosis. Nevertheless, not all available devices are equally effective, which makes it interesting to compare results in a preclinical model. Our objective was to assess the preclinical efficacy and safety of different devices.
MethodsWe implanted 51 metallic stents (Architect®, iVascular) in 17 domestic swine (mean, 25 [3] kg), inserting 1 stent per major coronary artery. Stent postdilatation was performed with different control balloons (n=10) or paclitaxel-eluting balloons: paclitaxel-eluting balloon 1 (iVascular) (n=15); paclitaxel-eluting balloon 2 (iVascular) (n=16) and In.Pact Falcon® (Medtronic) (n=10). The restenosis rate (using angiography and histomorphometry) and vascular healing parameters (balloon-related vascular injury score, endothelialization rate, and fibrin and inflammation scores) were analyzed at 28 days.
ResultsThe distinct paclitaxel-eluting balloons showed a similar degree of stenosis at follow-up, which was significantly lower than that in the control group: diameter stenosis was 9% (12%) vs 34% (18%) by angiography (P<.0001) and was 22% (8%) vs 51% (18%) by histomorphometry (P<.0001). Scores for vascular injury (mean, 0.6 [0.5]) and inflammation (mean, 0.8 [0.3]) were uniformly low across all groups. Drug effect markers differed significantly between the paclitaxel-eluting balloons and control groups, with lower endothelialization rates (87% [10%] vs 99% [2%]; P=.0007) and higher fibrin scores (2.1 [0.7] vs 0.4 [0.5]; P<.0001) in the paclitaxel-eluting balloons groups. There were no differences between the different paclitaxel-eluting balloons.
ConclusionsIn this preclinical model, the paclitaxel-eluting balloons studied significantly reduced in-stent restenosis compared with the control balloons. Although there were no findings of persistent vascular injury or inflammation, delayed endothelialization and fibrin aggregate suggest a drug deposition response.
Keywords
Coronary stents provide coating and luminal support, virtually eliminating the phenomenon of elastic recoil and subsequent negative vascular remodeling. As a result, the incidence of restenosis is 30% lower than in balloon angioplasty procedures.1,2 However, metallic stents do not reduce intimal hyperplasia and they also trigger hyperproliferation. Drug-eluting stents have virtually eliminated restenosis caused by neointimal proliferation.3,4 The drawback is that they have been found to delay and impair vascular healing,5–8 the clinical implications of which may be stent thrombosis5–7 and neoatherosclerosis.9,10 Permanent polymer coating has been associated with persistent inflammation, hypersensitivity, and deficient vascular healing in experimental models11,12 and human autopsy studies alike.5,6,13
To prevent these undesirable effects, alternative antiproliferative drug administration systems have been proposed, such as paclitaxel-eluting balloons (PEBs), which have proven efficacy in the prevention and treatment of restenosis in animal models14,15 and in clinical trials.16,17 However, not all currently-available PEBs yield the same results,18–22 and it would therefore be interesting to conduct a comparative analysis of various devices. This study aimed to compare the safety and efficacy results of various PEBs in the recommended23,24 porcine healthy coronary artery model, and to determine drug release kinetics and arterial drug deposition.
METHODSAnimal ModelIn this experimental, randomized, controlled study with blinded final sample analysis, we used 17 domestic large white pigs, aged 2-3 months, weighing 25 (3kg), from the experimental farm of our center. All procedures were carried out according to current Spanish regulations (Royal Decree 53/2013, of February 1, laying down the basic standards for the protection of animals used for experimental and other scientific purposes, including teaching) and European Directive 2010/63/EC. The local ethics committee approved the study protocol before we started any procedures.
All the pigs were given antiplatelet pretreatment with acetylsalicylic acid (325 mg) and clopidogrel (300 mg) 24h before the procedure. The anesthetic protocol and surgical preparation of the animals have been previously described in the literature.25,26 Briefly, the animals were prepared and then administered heparin 5000 IU intravenously. A left carotid artery approach was used to perform angiography in both coronary arteries, with prior administration of intracoronary nitroglycerin.
Coronary ProcedureWith the aim of implanting the devices to achieve a stent-to-artery ratio of 1.1 to 1.2, the best segment was located in each of the 3 major coronary arteries. After passing the angioplasty guidewire, a cobalt-chromium stent (Architect®, iVascular) was implanted in each major coronary artery. The stents were 14mm in length, with a diameter of 3.5mm (left anterior descending and right coronary artery) or 3mm (in the circumflex). We adjusted balloon inflation pressure to achieve the desired overstretching. After stent deployment, we performed postdilatation with various balloons, using the same diameter as the implanted stent and a length of 20mm, following a randomization table. We inflated the balloons at the manufacturers’ recommended nominal pressure for 1 or 2min (26 and 25 balloons, respectively) in a randomized manner, to analyze any differences in drug release by device type.
Devices AnalyzedThe following balloons were used (numbers in parentheses):
- 1.
Conventional plain balloon angioplasty control (n=10): Xperience® (iVascular).
- 2.
PEB 1 (n=15): experimental formulation 1 (iVascular). The Xperience® balloon is coated with paclitaxel (3μg/mm2 balloon surface) in a nanocrystalline formulation combined with a biocompatible plasticizer using TransferTech® ultrasonic deposition technology. This results in a homogeneous thin coating. The manufacturer estimates a theoretical drug release time of 30 to 60s, which means that balloon inflation for longer than 60s would not lead to any additional drug release.
- 3.
PEB 1 (n=16): experimental formulation 2 (iVascular). This is similar to PEB 1, with a more hydrophilic drug carrier matrix. The combination of the hydrophilic groups in this new matrix with hydrophobic groups already present in the backbone provides increased polarity in the coating, which potentially increases the solubility of the drug itself.
- 4.
PEB 3 (n=10): Marketed PEB In.Pact Falcon® (Medtronic). The paclitaxel formulation (3 μg/mm2 balloon surface) is also crystalline. The excipient, urea, is applied to the balloon using FreePac® technology.
All materials were supplied by iVascular, including PEB 1 and PEB 2, which are not yet available in the market. After the treatment had been applied, the balloons were then analyzed to determine the quantity of paclitaxel remaining in them, using high performance liquid chromatography.
Angiographic AnalysisAfter completing the above-described procedure on each artery, we then repeated the coronary angiography (with prior administration of intracoronary nitroglycerin) to determine the minimal luminal diameter in the stent. A control coronary angiography was performed at 28 days to determine the follow-up minimal luminal diameter. We measured the 2 variables and the reference diameters of the treated artery (mean diameter of the arterial segments located 5mm proximal and distal to the stent edges) using the automatic quantitative coronary analysis software Medis QCA-CMS ®, version 6.1. The following angiographic restenosis parameters were calculated:
Late loss = initial minimal luminal diamete – follow-up minimal luminal diameter
Angiographic percent diameter stenosis = [1 – (follow-up minimal luminal diameter/reference diameter)] × 100.
After completing the follow-up angiographic study, the animals were sacrificed and a full histologic study was performed. The hearts were excised and the coronary arteries were pressure-perfusion-fixed initially with phosphate buffered saline and then with 4% paraformaldehyde. The treated arterial segments were dissected, preserving at least 5mm proximally and distally. The samples were embedded in resin plastinated casts in order to take representative circumferential sections of the proximal, mid and distal segments of each sample, and to calculate mean values for each studied segment. After deplastinatng the sections, we performed routine hematoxylin-eosin and Van Gieson elastin staining procedures. To determine potential distant drug toxicity, we carried out a microscopic examination of key organs (lung, kidney, liver, spleen) and of the myocardium supplied by the treated arteries.
The arteries were analyzed histomorphometrically with digital microscopy imaging using an Olympus PRovis AX70® (Tokyo, Japan) with a Nikon DXM 1200® digital camera and the software ImageJ-NIH Image 1.4 (National Institutes of Health, United States). We used planimetry to determine the luminal and internal elastic lamina areas and calculate the 2 restenosis variables defined by histology:
Neointimal area = internal elastic lamina area – luminal area
Histologic percent diameter stenosis = [1 – (luminal area/internal elastic lamina area)] × 100.
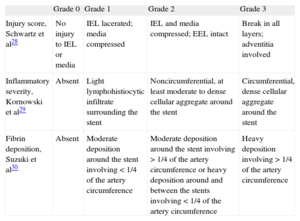
The histopathologic analysis of treatment safety was based on the semiquantitative analysis of the 4 main parameters27 shown in Table 1: injury score, defined by Schwartz et al,28 inflammatory severity, defined by Kornowski et al,29 persistent fibrin deposition by Suzuki et al30 and degree of (re-)endothelialization calculated from the approximate percentage of luminal surface covered by endothelial cells. In view of the large amount of surface covered by endothelial cells 28 days post-treatment, an additional parameter was established by defining complete reendothelialization as ≥ 95% of luminal surface covered by endothelial cells.27
Histopathologic Injury Score for Safety28
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
| Injury score, Schwartz et al28 | No injury to IEL or media | IEL lacerated; media compressed | IEL and media compressed; EEL intact | Break in all layers; adventitia involved |
| Inflammatory severity, Kornowski et al29 | Absent | Light lymphohistiocytic infiltrate surrounding the stent | Noncircumferential, at least moderate to dense cellular aggregate around the stent | Circumferential, dense cellular aggregate around the stent |
| Fibrin deposition, Suzuki et al30 | Absent | Moderate deposition around the stent involving <1/4 of the artery circumference | Moderate deposition around the stent involving >1/4 of the artery circumference or heavy deposition around and between the stents involving <1/4 of the artery circumference | Heavy deposition involving >1/4 of the artery circumference |
EEL: external elastic lamina; IEL: internal elastic lamina.
To identify the paclitaxel transfer rate to the artery, the quantity of drug remaining in the balloon and drug release into the bloodstream, an experimental phase was conducted on 3 additional animals with the following protocol: after performing selective catheterization of the left and right coronary arteries as described in the methodology section, we implanted cobalt-chromium stents in the proximal or medial segments of each major coronary branch to use as a marker. Distal to each stent, we dilated a PEB 1 balloon for 60 s at a pressure of 7 atm to 10 atm in order to achieve balloon-to-artery ratios of 1.1 to 1.2. Various follow-up time points were established (15-30 min, 2 balloons; 60-90 mins, 2; 2 h, 2; 24 h, 3), and after these time points the treated arteries were harvested for immediate freezing and subsequent analysis. Peripheral blood samples were also drawn at these time points.
Paclitaxel content was determined by means of high performance liquid chromatography. The treated arterial areas were processed by digesting the sample; the extracts were concentrated until dry and were redissolved in 1mL acetonitrile. After injecting the sample into the high performance liquid chromatography equipment (1260 Infinity HPLC-Chip/MS System, Agilent Technologies), paclitaxel was quantified by interpolation from the calibration curve and the results were expressed in micrograms of paclitaxel/gram of tissue. Flow rate was 0.8ml/min, using a Zorbax Eclipse Plus C18 column, 5 μm, 4.6×100 mm, with detection at 227 nm.
Statistical MethodValues were expressed as percentages and as mean (standard deviation), depending on the type of variable. Semiquantitative variables, such as safety variable scores determined by histopathology, were described as mean (standard deviation) (which is the most common way of presenting these data in previous publications) and as percentages (as recommended in consensus documents23,24).
We analyzed group mean differences using Student's t test and analysis of variance. For multiple comparisons, we performed a post-hoc analysis using Dunnett's comparison with a control and Tukey's all-pair comparison. Semiquantitative variables were analyzed using chi-square and Fisher's exact test. To assess the potential influence of different variables (stent-to-artery ratio, treated artery, inflation time and injury score) on the final results, we performed multivariate logistic regression analyses. A significant difference was defined as P<.05.
RESULTSAll stents were implanted and then overdilated with the balloons assigned by the protocol. Four transient complications occurred during the procedures, which were unrelated to the devices: 1 air embolism in the left coronary artery due to insufficient catheter purging, which we resolved with an amine infusion and 100% oxygen, and 3 cases (1 PEB 1, 1 PEB 2 and 1 control balloon) of distal coronary thrombosis caused by delayed or insufficient heparin administration, resolved with thrombectomy. None of the complications appeared to affect the implanted, overstretched devices, which remained patent and with a good flow. The oversized stent-to-artery dilatation ratio achieved in these phases attained the planned target of 1.21 (0.14), without significant differences between arteries or treatment groups. After harvesting the balloons posttreatment, only very small quantities of paclitaxel were found compared with the initial drug load: PEB 1: 1.04% (1.47%); PEB 2: 0.46% (0.56%), and PEB 3: 4.79% (3.40%), with no significant differences between them.
The animals completed the follow-up as planned, and the scheduled angiographic controls and histological analyses were performed with no incidents. All treated segments were patent in the final angiographic analysis. At necropsy, small areas of nontransmural infarction were observed in the 4 animals that developed complications during the procedure. The other cardiac specimens showed no significant abnormalities. Microscopic analysis of distant organs revealed no evidence of toxicity, hypersensitivity or other paclitaxel-related effect.
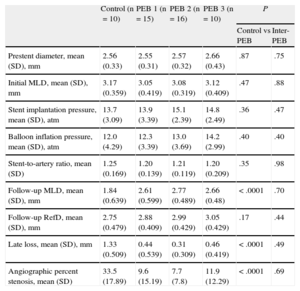
Antirestenosis Efficacy AnalysisSignificant differences (P<.0001) were observed between the control balloons and the different PEBs in terms of late loss (1.33 [0.5] vs 0.4 [0.42mm]) and also in angiographic percent diameter stenosis (33.5% [17.8%] vs 9.4% [11.8%]). There were no significant differences by PEB type or by inflation time (1 or 2 min) (Table 2 and Table 1 of supplementary material).
Initial and 28-day Angiographic Results
| Control (n=10) | PEB 1 (n=15) | PEB 2 (n=16) | PEB 3 (n=10) | P | ||
| Control vs PEB | Inter-PEB | |||||
| Prestent diameter, mean (SD), mm | 2.56 (0.33) | 2.55 (0.31) | 2.57 (0.32) | 2.66 (0.43) | .87 | .75 |
| Initial MLD, mean (SD), mm | 3.17 (0.359) | 3.05 (0.419) | 3.08 (0.319) | 3.12 (0.409) | .47 | .88 |
| Stent implantation pressure, mean (SD), atm | 13.7 (3.09) | 13.9 (3.39) | 15.1 (2.39) | 14.8 (2.49) | .36 | .47 |
| Balloon inflation pressure, mean (SD), atm | 12.0 (4.29) | 12.3 (3.39) | 13.0 (3.69) | 14.2 (2.99) | .40 | .40 |
| Stent-to-artery ratio, mean (SD) | 1.25 (0.169) | 1.20 (0.139) | 1.21 (0.119) | 1.20 (0.209) | .35 | .98 |
| Follow-up MLD, mean (SD), mm | 1.84 (0.639) | 2.61 (0.599) | 2.77 (0.489) | 2.66 (0.48) | <.0001 | .70 |
| Follow-up RefD, mean (SD), mm | 2.75 (0.479) | 2.88 (0.409) | 2.99 (0.429) | 3.05 (0.429) | .17 | .44 |
| Late loss, mean (SD), mm | 1.33 (0.509) | 0.44 (0.539) | 0.31 (0.309) | 0.46 (0.419) | <.0001 | .49 |
| Angiographic percent stenosis, mean (SD) | 33.5 (17.89) | 9.6 (15.19) | 7.7 (7.8) | 11.9 (12.29) | <.0001 | .69 |
MLD, minimal luminal diameter in the stent; PEB: paclitaxel-eluting balloon; RefD, reference diameter; SD, standard deviation.
Data are expressed as mean (standard deviation).
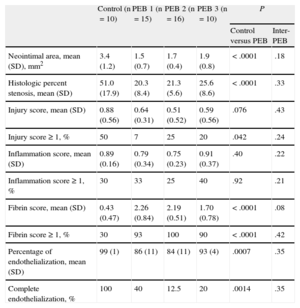
All arterial segments were analyzed appropriately by histomorphometry, covering a total of 153 sections. Coinciding with the angiographic findings, restenosis results were also better for the different PEBs than for the control balloons (P<. 0001) by histomorphometry. No significant differences were observed among the PEB types with respect to inflation time (Table 3, Table 2 of supplementary material, and Figure 1).
Histology Results at 28 Days
| Control (n=10) | PEB 1 (n=15) | PEB 2 (n=16) | PEB 3 (n=10) | P | ||
| Control versus PEB | Inter-PEB | |||||
| Neointimal area, mean (SD), mm2 | 3.4 (1.2) | 1.5 (0.7) | 1.7 (0.4) | 1.9 (0.8) | <.0001 | .18 |
| Histologic percent stenosis, mean (SD) | 51.0 (17.9) | 20.3 (8.4) | 21.3 (5.6) | 25.6 (8.6) | <.0001 | .33 |
| Injury score, mean (SD) | 0.88 (0.56) | 0.64 (0.31) | 0.51 (0.52) | 0.59 (0.56) | .076 | .43 |
| Injury score ≥ 1, % | 50 | 7 | 25 | 20 | .042 | .24 |
| Inflammation score, mean (SD) | 0.89 (0.16) | 0.79 (0.34) | 0.75 (0.23) | 0.91 (0.37) | .40 | .22 |
| Inflammation score ≥ 1, % | 30 | 33 | 25 | 40 | .92 | .21 |
| Fibrin score, mean (SD) | 0.43 (0.47) | 2.26 (0.84) | 2.19 (0.51) | 1.70 (0.78) | <.0001 | .08 |
| Fibrin score ≥ 1, % | 30 | 93 | 100 | 90 | <.0001 | .42 |
| Percentage of endothelialization, mean (SD) | 99 (1) | 86 (11) | 84 (11) | 93 (4) | .0007 | .35 |
| Complete endothelialization, % | 100 | 40 | 12.5 | 20 | .0014 | .35 |
PEB: paclitaxel-eluting balloon; SD, standard deviation.
Unless otherwise indicated, the data are expressed as mean (standard deviation).
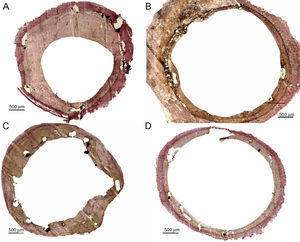
Central sections of different stents overdilated with different balloons, Van Gieson elastin staining. A: control balloon; significant neointimal growth, no evidence of deep inflammation, uniform endothelial coverage. Paclitaxel drug-eluting balloons, B: balloon 1 (iVascular); C: balloon 2 (iVascular), and D: balloon 3 (In.Pact Falcon, Medtronic): slight neointimal growth, no evidence of deep inflammation, acceptable endothelialization.
The statistical analyses were repeated excluding the arteries involved in the events mentioned earlier. There was no significant variation in the results. We performed multivariate analyses to assess the potential influence of different known factors on the observed values. We found an independently lower degree of restenosis in right coronary artery vs left anterior descending (P<.02), and in any PEB vs control (P<.01) across all models, with no differences by PEB type.
Safety AnalysisThe histologic analysis revealed a low injury score in general, without significant differences between the groups (control, 0.88 [0.56] vs all PEBs, 0.58 [0.46]; P=.07). The same was true for the inflammation score, which was very low in general (control, 0.89 [0.16] vs all PEBs, 0.80 [0.31]; P=.4). No cases were observed of media necrosis. However, 2 significant differences were noted that are characteristic of paclitaxel release (Figure 2): a) persistent fibrin deposition was significantly greater in the PEBs (2.09 [0.73], with no differences by PEB type) than in the control group (0.43 [0.47]; P=.0001), and b) the percentage of endothelialized luminal surface was significantly lower in the PEBs (87% [10%], with no significant differences by PEB type) than in the control group (99% [1%]; P=.0007). Complete reendothelialization (endothelial coverage ≥ 95%) was observed in 100% of the control balloons vs 40% in PEB 1, 12.5% in PEB 2, and 20% in PEB 3.
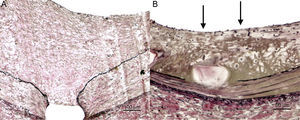
Detail of arterial response, Van Gieson elastin staining. A: control balloon; significant neointimal growth, complete endothelialization of luminal surface, no evidence of inflammation or fibrin deposition. B: Paclitaxel drug-eluting balloon balloon 1 (iVascular); less neointimal growth, acceptable but incomplete luminal endothelialization (arrows) and significant deep fibrin deposition around the stent (brownish coloring).
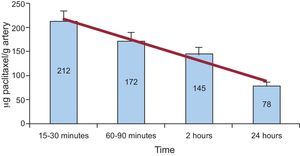
In our study, paclitaxel concentrations found in the treated arteries decreased progressively from the first determinations at 15 to 30 min with 212 (32) μg/g tissue, through 24h, with 78 (9) μg/g tissue still present (Figure 3). Paclitaxel was not detectable in peripheral blood at any time point.
DISCUSSIONThe main results of this study are: a) the PEBs used in this preclinical model equally and significantly reduced in-stent restenosis, as corroborated by the angiographic and histologic analyses; b) they did not show a more persistent inflammatory reaction or have a higher vascular injury score than the control balloons, and c) the effective drug release from these devices caused a delay in vascular healing compared with the control group, as evidenced by the lower reendothelialization rate and more persistent deep fibrin deposition around the stent.
All antirestenosis efficacy parameters studied consistently demonstrate the superiority of the different PEBs vs the plain control. Previous studies have already shown the superior efficacy of different marketed PEBs vs control balloons,14,15 although in some cases the differences were not evident in all parameters analyzed.20,21 The model we used was very similar to previous ones with a 28-day follow-up (equivalent to 6 months in humans), and our oversized dimension parameters (stent-to-artery ratio, 1.21 [0.14]) and percent diameter stenosis by angiography (33.5% [17.8%]) and by histology (51% [17.9%]) in the control group are similar to studies mentioned earlier.14,15,20,26 In our series there were no differences by PEB type or by inflation time, which was equal to or greater than the manufacturers’ recommendations. These results are corroborated by the multivariate analyses that we used to limit the influence of potential confounders.
Despite substantial overstretching, the vascular injury score is not very high, with a mean value of 0.64 [0.49] (12 of the 51 stents had a mean score of >1, but none reached a score of 2). There were no differences by PEB type, but we observed a tendency towards greater vascular injury in the control group. This finding was not confirmed in the multivariate analysis, which only showed a relationship between the injury score and the stent-to-artery ratio, which was slightly higher in the control groups (1.25 [0.16]) than in the PEB groups (1.20 [0.14]). No between-group differences were noted in the degree of persistent inflammation, with low values in all cases (media, 0.82 [0.29]). This finding rules out toxicity phenomena, which is consistent with the absence of significant media necrosis, hypersensitivity, and allergy. This response may be explained by the lack of polymers in many drug-eluting stents. Polymers may prolong these abnormal vascular healing phenomena.5,6,11–13
However, we did observe 2 phenomena associated with delayed vascular healing that are directly related to the antiproliferative effect of paclitaxel: reendothelialization was slightly but significantly lower in the PEBs than in the control, and fibrin deposition around the stent was also significantly higher in the PEBs than in the control. This observation matches previous findings by Joner et al,20 who showed that only PEBs that effectively reduced restenosis presented a lower degree of reendothelialization and a higher amount of fibrin deposition. These observations should be interpreted as proof of concept of the effective transfer of paclitaxel to the artery. Some previous studies have described adequate 28-day endothelialization of stents overdilated with PEBs,14,15 although it should be noted that post treatment arterial reendothelialization is challenging to assess, as reflected by the absence of a single classification system for this purpose.27 In any case, the percentage of endothelialized surface of stents overdilated with PEB ranges from 84% [3%] to 95% [4%], which is very similar to previous descriptions of antirestenosis efficacy in PEBs.20,21
There is a very high drug transfer rate from the PEBs because less than 10% of the initial drug load in the balloon remains in the balloon post-procedure. Paclitaxel concentrations measured in our study show that the drug persists in the arterial wall for up to 24h after administration, which explains why a very short drug application time can produce longer-term effects. These drug concentrations are very similar to those observed in other studies,31 which report a linear reduction in concentrations from balloon deployment through 24h, and persisting concentrations with largely unchanging values for up to 28 days.
LimitationsThis series, like all preclinical models, has inherent limitations, because no animal model can reproduce all the complex characteristics of human disease. Diseased animal models might reflect some of these characteristics, but their exact interpretation is still unclear. The general consensus is to use the same model that we used in this study to analyze vascular response to this type of device.23,24 This specific model of coronary stenting and subsequent overstretching with a balloon does not accurately reflect all the clinical conditions in which these devices are used, but it does represent the best experimental model to induce restenosis, as evidenced by its use in the literature.14,15,20,21,31 This model shares the characteristics of de novo lesions and restenosis. The controversial reduced efficacy of PEBs in the former could help explain the lack of differences among the 3 PEBs. Although our results show no differences by PEB type, such differences cannot be ruled out in other designs not included in this study.20,22 Histologic assessment using semiquantitative variables also limits accuracy, but we followed the consensus scoring systems in this respect.23,24 The model used for the pharmacokinetic study may also be questioned because it does not take into account the significant role that atherosclerotic plaque can play in drug uptake.
CONCLUSIONSIn the preclinical normal porcine coronary model with stent deployment and overstretching with a balloon, the PEBs studied showed significant reduction in restenosis compared with controls. Although there was no evidence of persistent vascular injury or inflammation, delayed endothelialization and deep fibrin aggregate suggest a drug deposition response.
FUNDINGThis study was partly developed with the support of CDTI (Centro para el Desarrollo Tecnológico Industrial, Ministerio de Economía y Competitividad, Center for Industrial Technology Development, Ministry of Economy and Competitiveness) IDI-20111025.
CONFLICTS OF INTERESTDr. Armando Pérez de Prado and Dr. Felipe Fernández-Vázquez are consultants and receive nonspecific support for conducting various research projects at LVD Biotech/iVascular. María Molina, Alex Gómez, and Luis Duocastella are employed at LVD Biotech/iVascular. Luis Duocastella has a shareholding in LVD Biotech/iVascular. The other authors have no conflicts of interest to declare.