To assess, in patients with ST-segment elevation myocardial infarction (STEMI) who underwent primary percutaneous intervention, the pace of introduction in clinical practice (2010-2017) of drug-eluting stents (DES), ticagrelor, prasugrel, and prolonged dual antiplatelet therapy (DAPT) duration, and their potential impact on the risk of 2-year outcomes.
MethodsProspective and exhaustive community-wide cohort of 14 841 STEMI patients undergoing primary percutaneous intervention between 2010 and 2017. Index episodes were obtained from the Catalan Codi IAM Registry, events during follow-up from the Minimum Data Set and DAPT were defined by pharmacy dispensation. Follow-up was 24 months. The temporal trend for exposures and outcomes was assessed using regression models.
ResultsAge> 65 years, diabetes, renal failure, previous heart failure, and need for anticoagulation at discharge were more frequent in later periods (P <.001). From 2010 to 2017, the use of DES increased from 31.1% to 69.8%, ticagrelor from 0.1% to 28.6%, prasugrel from 1.5% to 23.8%, and the median consecutive months on DAPT from 2 to 10 (P <.001 for all). Adjusted analysis showed a temporal trend to a lower risk of the main outcome over time: the composite of death, acute myocardial infarction, stroke and repeat revascularization (absolute odds reduction 0.005% each quarter; OR, 0.995; 95%CI, 0.99-0.999; P=.028). The odds of all individual components except stroke were reduced, although significance was only reached for revascularization.
ConclusionsDespite a strong increase between 2010 and 2017 in the use and duration of DAPT and the use of ticagrelor, prasugrel and DES, there was no substantial reduction in major cardiovascular outcomes.
Keywords
Although the decrease in mortality rates in patients with ST-segment elevation myocardial infarction (STEMI) has been attributed basically to the benefits of early reperfusion,1,2 other concomitant therapies for STEMI have also achieved the highest level of recommendation in clinical guidelines during the last decade. Thus, the use of drug-eluting stents (DES) for primary percutaneous coronary intervention (PCI), the use of the P2Y12 inhibitors ticagrelor and prasugrel, and the maintenance of dual antiplatelet therapy (DAPT) for at least the first 12 months, in the absence of contraindications, have become routine over time.3–5
Although these high-level evidence recommendations in clinical guidelines are based on large randomized clinical trials,6-10 few studies have analyzed the pace of their introduction in clinical practice and their overall impact on mid-term outcomes in patients with revascularized STEMI.11 Currently, given that adherence to clinical guidelines is not inmediate, a delay in their potential benefit would be expected when these recommendations are introduced in clinical practice.11,12
The Codi IAM registry has prospectively colleted data since 2010 from an exhaustive cohort of patients with STEMI in Catalonia, an autonomous region of Spain.13–16 It is estimated that the registry includes more than 90% of all STEMI cases in Catalonia contacting the health system.13 The data collection and data management in Codi IAM has remained practically unchanged over time, which may avoid certain biases. In addition, linking the data from the Codi IAM network registry to the Minimum Data Set (MDS) for hospitalization episodes, mortality records and prescription refills, allows the assessment of the occurrence of outcomes over time and of their relationship with the introduction of specific therapies in clinical practice. In this study, we evaluated, in patients with STEMI who underwent primary PCI and were discharged alive, the pace of introduction in clinical practice since 2010 of four interventions of interest (ie, DES, ticagrelor, prasugrel, and prolonged DAPT duration) and the potential relationship between the change over time in the extent of use of the specified interventions and subsequent 2-year outcome rates.
METHODSStudy design and data sourcesThis is a retrospective analysis of data from patients prospectively included in a specific AMI clinical registry, linked to administrative data for pharmacy prescription, MDS, and mortality registries.
Data were obtained through the Public Data Analysis for Health Research and Innovation (PADRIS) program. The program allows access to information from different sources on public health care usage for the population of Catalonia and is linked at the patient level with warranted accomplishment of ethical principles and the Spanish Law of data protection 3/2018. The study protocol was approved by the Vall d’Hebron ethics committee and there was no requirement for patients’ informed consent.
Eligibility and baseline clinical information was obtained from the Codi IAM network registry. This registry was launched in 2010 to evaluate the performance of the Codi IAM emergency reperfusion network, consisting of the activation of a planned set of coordinated actions aimed at treating patients with STEMI with the best timely therapeutic alternative.13,16 The database comprising the registry belongs to the Catalan Health Department and includes demographic, clinical, and therapeutic data. It conforms to the ethical and legal requirements for research purposes. All study procedures were in accordance with the ethical standards outlined in the Helsinki Declaration. The quality of the data included in the registry is periodically verified by means of an external audit. Although the registry was launched in 2010, several variables were incorporated in subsequent years. For the present analyses, we only considered those variables that were in the registry since the beginning in 2010.
Events during follow-up were obtained from the MDS (table 1 of the supplementary data), while death was obtained from the Mortality Registry. Drug treatment during follow-up was obtained from the database of pharmacy prescription refills, which registers all pharmacy products and the number of containers dispensed to each individual on a monthly basis.
Inclusion criteriaWe included all patients registered in the Codi IAM network database surviving the index episode (who were discharged alive or who were alive 2 weeks after STEMI) and who underwent primary PCI, with available data for a minimum of 2 years of follow-up. We excluded those patients without complete information on treatment during follow-up.
Exposure definitionWe quantified the extent of introduction in clinical practice since 2010 of 4 interventions of interest: DES implantation during the index procedure, ticagrelor prescription at discharge, prasugrel prescription at discharge, and consecutive months on DAPT.
The use of DES was obtained from the Codi IAM registry or the MDS (diagnostic code 36.07 for DES and 36.06 for bare metal stent), while the use of ticagrelor, prasugrel and the time on DAPT was obtained from the prescription refill. DAPT was defined as the concomitant use of aspirin and a P2Y12 inhibitor each month after the index episode. Use of medication was defined as dispensation of 1 container of each agent for each month after hospital discharge. If more than 1 container was dispensed in 1 month, the excess containers were carried forward during the following months filling the gaps between dispensations and until the last dispensation. DAPT period was defined as the period with continuous monthly dispensation, allowing a lag of 2 months between 2 dispensations.
Outcomes definitionThe primary endpoint was a composite endpoint defined as the occurrence of either death from any cause, acute myocardial infarction (AMI), ischemic stroke, or revascularization after the index episode and within the first 2 years after discharge. AMI and ischemic stroke during follow-up were defined as a hospitalization episode occurring after discharge for the index procedure with a primary diagnosis of AMI and ischemic stroke, respectively. Major hemorrhages were defined as a diagnosis of hemorrhagic stroke or intraocular hemorrhage or a diagnosis of gastrointestinal bleeding or other types of hemorrhage that required invasive treatment or transfusion of blood products. See table 1 of the supplementary data for the definition of events from the International Classification of Diseases 9 and 10 codes. Follow-up was truncated at 24 months after the index episode, to ensure a homogeneous time-exposure.
Statistical analysisCategorical variables are described using absolute and relative frequencies. Numerical variables are described as mean±standard deviation, or as median and interquartile range. The distribution of all variables was compared between 2-year blocks (ie, 2010-2011, 2012-2013, 2014-2015, 2016-2017). Temporal trends during periods in categorical and continuous variables were assessed using a nonparametric test for trend across ordered groups (an extension of the Kruskal-Wallis test).
Outcome event analysis was based on patients (ie, number of patients with at least 1 event during the 2-year follow-up) and on the total event-rate (ie, number of total events per 1000 patients-years of follow-up). The following events were analyzed: all-cause death, myocardial infarction, stroke, revascularization, major hemorrhage, and the composite of death, AMI, stroke, and revascularization.
The temporal trend for exposures of interest and outcomes was assessed using regression models. We used linear, logistic and Poisson modeling for continuous, discrete, and incidence rate dependent variables, respectively. The magnitude of the temporal trend was assessed using different reference time periods such as weeks, months, quarters, and years. Linear, quadratic and spline trends were tested. We adjusted 3 sequential multivariable models to estimate the temporal trends while adjusting for changes in patient characteristics over time. The “empty” model (M0) included only the time period (weeks, months, quarters, years) to test for the temporal lineal trend. In model 1 (M1), we added age (continuous) and sex. In model 2 (M2), we added all risk factors and comorbidities available from the registry in the whole analysis period: renal impairment, previous AMI, diabetes, Killip III-IV, and anterior location of the myocardial infarction. Finally, as a sensitivity analysis, we reanalyzed all models excluding those patients with anticoagulation therapy at discharge.
We used R version 4.0.4 for all statistical analyses. A P value <.05 was considered significant.
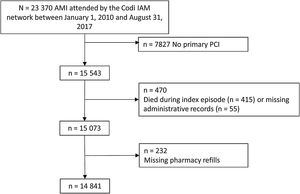
RESULTSA total of 23 370 patients with suspected STEMI were treated within the Codi IAM network between January 1, 2010 and August 31, 2017. After exclusion of those patients who did not undergo primary PCI (n=7827, approximately one half due to inappropriate activations and the rest due to final diagnoses other than AMI, lack of significant culprit lesions or other reasons), who died during the index episode (n=415), with missing administrative information (n=55), or with missing pharmacy refills (n=232), there were 14 841 patients with valid data for the analyses (figure 1).
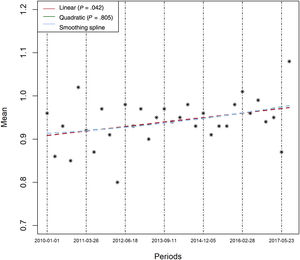
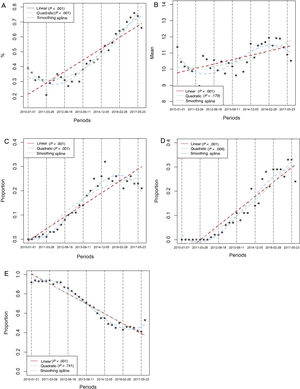
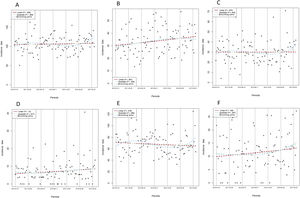
Table 1 compares the baseline characteristics and the interventions of interest between 2-year blocks. Mean age significantly increased over time (P=.004), as did the prevalence of diabetes (P=.042), renal impairment (P <.001), and the need of anticoagulation at discharge. By contrast, a history of prior AMI decreased over time. There were no important differences in other baseline risk variables, although there was a trend to a higher rate of Killip III/IV class (P=.076) and a higher prevalence of anterior AMI (P=.072) in later periods. Regression analysis confirmed a clear linear increase over time in the mean number of the following risk factors per patient: age> 65 years, diabetes, renal failure, previous heart failure, previous AMI, and need for anticoagulation at discharge (figure 2, P for linear trend=.0042), which suggests a higher risk population in later periods. The use of DES during primary PCI and prescription at discharge of ticagrelor, prasugrel and clopidogrel were markedly different between 2-year blocks (table 1). Graphic temporal trend analyses confirmed a strong increase in the use of DES, along with an increase in the average duration of DAPT and an increase in the use of prasugrel and ticagrelor rather than clopidogrel (figure 3 and figure 1 of the supplementary data).
Population and intervention differences between 2-year time periods
| 2010-2011(n=3362) | 2012-2013(n=3755) | 2014-2015(n=4060) | 2016-2017(n=3664) | P* | All(n=14 841) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean±SD | 62.21±13.17 | 62.62±12.86 | 62.74±13.01 | 63.3±12.96 | .004 | 62.73±13 |
| Median [IQR] | 52 [62-73] | 53 [62-73] | 53 [61-73] | 53 [62-73] | 62 [53-73] | |
| [Min-max] | [24-93] | [24-98] | [29-96] | [27-98] | [24-98] | |
| Female sex | 683 (20.3) | 795 (21.2) | 848 (20.9) | 795 (21.7) | .216 | 3121 (21) |
| Previous AMI | 289 (8.6) | 313 (8.3) | 295 (7.3) | 240 (6.6) | <.001 | 1137 (7.7) |
| Diabetes | 665 (19.8) | 739 (19.7) | 744 (18.3) | 816 (22.3) | .042 | 2964 (20) |
| Previous PCI | 203 (6) | 242 (6.4) | 263 (6.5) | 229 (6.2) | .728 | 937 (6.3) |
| Previous CABG | 28 (0.8) | 37 (1) | 50 (1.2) | 34 (0.9) | .478 | 149 (1) |
| Renal impairment | 208 (6.2) | 231 (6.2) | 333 (8.2) | 315 (8.6) | <.001 | 1087 (7.3) |
| Chronic obstructive pulmonary disease | 343 (10.2) | 401 (10.7) | 457 (11.3) | 386 (10.5) | .500 | 1587 (10.7) |
| Heart failure history | 584 (17.4) | 697 (18.6) | 768 (18.9) | 643 (17.5) | .782 | 2692 (18.1) |
| Peripheral arterial disease | 102 (3) | 127 (3.4) | 147 (3.6) | 125 (3.4) | .317 | 501 (3.4) |
| Killip III-IV | 175 (5.2) | 223 (5.9) | 233 (5.7) | 232 (6.3) | .076 | 863 (5.8) |
| Anterior AMI | 1308 (38.9) | 1458 (38.8) | 1658 (40.8) | 1480 (40.4) | .072 | 5904 (39.8) |
| Anticoagulation at discharge | 156 (4.6) | 211 (5.6) | 244 (6) | 249 (6.8) | .014 | 860 (5.8) |
| Interventions | ||||||
| Drug-eluting stent | 1046 (31.1) | 1231 (32.8) | 1991 (49) | 2556 (69.8) | <.001 | 6824 (46) |
| Clopidogrel prescribed at discharge | 3115 (92.7) | 3043 (81.0) | 2216 (54.6) | 1640 (44.8) | <.001 | 10 014 (67.5) |
| Prasugrel prescribed at discharge | 51 (1.5) | 374 (10) | 992 (24.4) | 871 (23.8) | <.001 | 2288 (15.4) |
| Ticagrelor prescribed at discharge | 3 (0.1) | 184 (4.9) | 713 (17.6) | 1047 (28.6) | <.001 | 1947 (13.1) |
| Months on DAPT | 2 [11-15] | 3 [11-14] | 7 [11-13] | 10 [12-14] | <.001 | 11 [5-14] |
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention.
The results are expressed as No. (%), mean±standard deviation, median [interquartile range], or [min-max].
Temporal trends in the interventions of interest over time. A: rate of use of drug-eluting stent in primary PCI. B: mean number of consecutive months with DAPT. C: proportion of patients with prasugrel prescription at discharge. D: proportion of patients with ticagrelor prescription at discharge. E: proportion of patients with clopidogrel prescription at discharge. Temporal trends were tested in linear, quadratic and smoothing spline models. The time unit reference is the quarter. Other time unit references were performed. See figure 1 of the supplementary data. DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention. * Observed values.
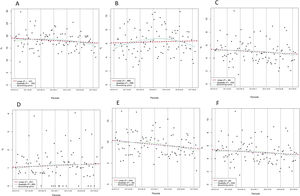
Table 2 compares outcome rates between 2-year blocks. There were significant differences between 2-year blocks only for the total number of patients with at least 1 revascularization (P=.024). However, the incidence rate of revascularization, which expresses the total number of revascularizations, was also similar between time periods (table 2). Graphic temporal trend analyses did not show a statistically significant change over time in outcomes except for the number of patients with at least 1 revascularization, which decreased over time (P=.043) (figure 4). However, the incidence rate of revascularization showed no change over time (P=.278) (figure 5).
Differences between time periods in 2 year outcomes
| 2010- 2011(n=3362) | 2012-2013(n=3755) | 2014-2015(n=4060) | 2016-2017(n=3664) | P | |
|---|---|---|---|---|---|
| Number of patients with at least 1 outcome | |||||
| Composite endpoint of death, AMI, stroke, revascularization | 620 (18.4) | 717 (19.1) | 702 (17.3) | 638 (17.4) | .076 |
| All death | 200 (5.9) | 243 (6.5) | 260 (6.4) | 226 (6.2) | .781 |
| AMI | 168 (5) | 205 (5.5) | 191 (4.7) | 170 (4.6) | .23 |
| Stroke | 38 (1.1) | 38 (1) | 52 (1.3) | 41 (1.1) | .738 |
| Revascularization | 368 (10.9) | 427 (11.4) | 399 (9.8) | 359 (9.8) | .024 |
| Major Bleeding | 62 (1.8) | 65 (1.7) | 76 (1.9) | 76 (2.1) | .395 |
| Outcome incidence rate (1000 pts-year) | |||||
| Composite endpoint of death, AMI, stroke, revascularization | 130 (121-139) | 139 (131-148) | 126 (118-134) | 138 (129-148) | .656 |
| All death rate | 31.1 (27.1-35.6) | 34.0 (30.0-38.4) | 33.9 (30.0-38.1) | 36.2 (31.8-41.1) | .15 |
| AMI rate | 28.5 (24.6-32.7) | 30.9 (27.1-35.2) | 27.3 (23.9-31.2) | 29.9 (26.0-34.4) | .983 |
| Stroke rate | 6.7 (5.0-8.8) | 5.6 (4.1-7.5) | 7.4 (5.7-9.5) | 8.8 (6.8-11.3) | .080 |
| Revascularization rate | 63.6 (57.8-69.9) | 68.3 (62.5-74.5) | 57 (51.9-62.5) | 63.2 (57.3-69.6) | .310 |
| Major bleeding rate | 11.2 (8.8-14.1) | 10.2 (8.0-12.9) | 11.3 (9.1-14.0) | 13.0 (10.3-16.1) | .283 |
AMI, acute myocardial infarction.
The results are expressed as No. (%) or 95% confidence intervals.
Table 3 shows the time trend for 2-year outcomes in the unadjusted and the adjusted analyses. On adjustment for age and sex (M1), there was a clear temporal trend to a lower risk of the composite endpoint of all-cause death, AMI, stroke, and revascularization (absolute odds reduction 0.005% each quarter; odds ratio [OR], 0.995; 95% confidence interval [95%CI], 0.99-1) that persisted after adjustment for renal impairment, previous AMI, diabetes, Killip III/IV class, and anterior STEMI location (P=.028) (M2). Although nonsignificant, we found a similar trend in the same direction for the individual components of the composite outcome: death, AMI and specially, revascularization. Finally, risk of major bleeding did not change over time. The unadjusted and adjusted time-trend models excluding those patients with anticoagulation prescribed at discharge (n=860) showed similar results (table 2 of the supplementary data).
Outcomes time-trend in the unadjusted and adjusted analyses in the whole population
| OR (95%CI) | P | |
|---|---|---|
| Death or AMI or stroke or revascularization | ||
| M0 | 0.996 (0.992-1.001) | .121 |
| M1 | 0.995 (0.99-1) | .045 |
| M2 | 0.995 (0.99-0.999) | .028 |
| Death | ||
| M0 | 1.002 (0.995-1.01) | .544 |
| M1 | 0.998 (0.991-1.006) | .706 |
| M2 | 0.996 (0.988-1.004) | .335 |
| AMI | ||
| M0 | 0.995 (0.986-1.003) | .22 |
| M1 | 0.994 (0.986-1.003) | .198 |
| M2 | 0.995 (0.986-1.003) | .215 |
| Stroke | ||
| M0 | 1.003 (0.986-1.02) | .755 |
| M1 | 1 (0.983-1.018) | .967 |
| M2 | 1 (0.983-1.018) | .971 |
| Revascularization | ||
| M0 | 0.993 (0.987-1) | .038 |
| M1 | 0.994 (0.988-1) | .058 |
| M2 | 0.995 (0.988-1.001) | .089 |
| Bleeding | ||
| M0 | 1.007 (0.994-1.021) | .282 |
| M1 | 1.006 (0.992-1.019) | .416 |
| M2 | 1.004 (0.991-1.018) | .528 |
95%CI, 95% confidence interval; AMI, acute myocardial infarction;
M0: Lineal trend of the time unit (quarter). M1: M0 + age + sex. M2: M1 + renal impairment + previous AMI + diabetes + KILLIP III/IV + anterior AMI; OR, odds ratio.
Time unit is the quarter. Models express the decrease or increase in the risk each quarter
The main finding of this large temporal trend outcomes analysis in patients with a revascularized STEMI is that, despite a strong increase between 2010 and 2017 in the use of DES during primary PCI, in the use of ticagrelor and prasugrel instead of clopidogrel, and in the median time on DAPT after discharge, the change in major cardiovascular outcomes at 2-year follow-up was limited. We observed a significant temporal decrease in the composite of death, AMI, stroke or revascularization, but, although there was a decrease of similar magnitude in all the individual components except stroke, it was only statistically significant for repeat revascularization.
We could not demonstrate a clear risk-adjusted temporal change in relevant individual outcomes in revascularized patients who survived to the index episode, despite the substantial increase over time in the use of DES, prasugrel, ticagrelor, and more prolonged DAPT therapy.
Other studies that have analyzed temporal trends in AMI management and outcomes have demonstrated an increase in the prevalence of evidence-based treatment that was associated with a sustained decrease in major outcomes during follow-up. However, most of these studies probably captured the impact of the generalization of early reperfusion in STEMI,11 which was not the focus of our study. The analysis of the SWEDHEART registry by Szummer et al.17 shows how the most powerful changes in clinical outcomes of AMI were driven by the reductions of ischemic times due to the progressive expansion of primary PCI in the decade 2000 to 2010. The use of secondary prevention treatments provided further but weaker improvements, while concomitant treatment to PCI (cardioprotective therapies aiming to reduce reperfusion injury) has overall failed to provide a benefit.18
There could be several reasons that explain the absence of substantial changes in major outcomes in these patients despite the relevant improvement in the adoption of these guideline-recommended therapies.
First, the actual effect of these therapies in the real-world clinical setting could be lower than expected from the results of their corresponding pivotal clinical trials,6–10,19 at least in STEMI patients revascularized with primary PCI. On the other hand, it cannot be ruled out that with greater use of prasugrel and ticagrelor instead of clopidogrel, greater reductions in ischemic events would be observed. Regarding DES, clinical trials have consistently found a reduction in the rates of repeat coronary revascularization but have differed in the effects of DES on major endpoints.6,7,19 Regarding antiplatelet agents, neither prasugrel nor ticagrelor significantly reduced the combined endpoint of cardiovascular death, reinfarction, or stroke over clopidogrel in analyses of the subset of patients with STEMI undergoing primary PCI included in the respective pivotal trials.20,21 Regarding optimal DAPT duration, this is still a matter of debate, particularly in patients with revascularized STEMI, in whom the maximal risk of recurrent ischemic events is concentrated in the first months after the infarction.5,22
Second, the time-window in our study might be too narrow to observe a benefit of the gradual adoption of these therapies; a higher rate of use might be necessary to translate the benefit observed in clinical trials to the real world. In this regard, the only outcome that decreased over time was the number of patients with new revascularizations, which is probably the most relevant effect associated with the use of DES,6,7,19 the exposure showing the greatest increase over time in our cohort.
Third, a 2-year follow-up might be insufficient to observe the potential benefit of these therapies on major outcomes. In this regard, in the EXAMINATION trial, a benefit of DES on all-cause mortality was only observed at 5 years of follow-up.7 In our study, in the adjusted analyses, both all-cause death and AMI showed a nonsignificant trend to a lower risk over time (OR, 0.996; 95%CI, 0.988-1.004 and 0.995; 95%CI, 0.986-1.003 respectively), which suggest that the study might be underpowered. Irrespective of this consideration, the absence of statistical significance in the risk reduction of these outcomes in the analysis of more than 14 000 patients followed up for 2 years argues against major changes achieved in the prognosis of the revascularized STEMI from 2010 to 2017. Noticeably, a sensitivity analysis excluding patients on oral anticoagulation, who represent a especial population with regard to their higher event risk and their management, obtained similar results than those observed in the overall cohort.
In our cohort, we observed that STEMI patients tended to be older and to have more comorbid conditions such as diabetes and renal impairment over time. This finding is in contrast with those of other studies.11,23 Although an influence of the demographic structure of the Spanish population cannot be ruled out, since Spain is one of the western countries with the highest increase in the mean age of the population, comparisons with other contemporary STEMI populations should be avoided24,25 because we selected those patients who underwent primary PCI and who had survived the index episode. In this regard, the improvement in the short-term prognosis of STEMI secondary to primary PCI is fully documented in real world registries,11 while there is a lack of studies assessing the temporal trend outcomes in the mid- to long-term in revascularized patients, which is currently the most frequent situation in western countries.
One of the findings of the present study is that these high-level evidence-based therapies have been gradually incorporated into clinical practice. This has also been observed previously11 and probably reflects variations in the process of care, which highlights the need for continuous programs to homogenize and standardize health care. Rigorous measurement, reporting and benchmarking of process and outcomes indicators, such as those recently updated by the European Society of Cardiology,26 are fundamental for quality improvement. In the specific case of the use of DES, prasugrel and ticagrelor, an influence of the pharmaceutical budgetary restrictions due to the economic crisis, which was especially virulent in Spain, cannot be ruled out as a cause of the slow introduction of newer and more expensive therapies in clinical practice.
Although the introduction of ticagrelor, prasugrel and prolonged DAPT duration in clinical practice in our context has been gradual, between 2010 and 2017 the number of patients with ticagrelor and prasugrel has multiplied 10-fold and the mean number of consecutive months on DAPT per patient has increased by more than 20%. Despite this, the number of patients who have had an episode of major bleeding and the major bleeding rate have remained virtually unchanged since 2010, even considering that some bleeding risk factors have become more prevalent (ie, age> 65 years, diabetes, renal impairment). This may indicate that both ticagrelor and prasugrel do not substantially increase the risk of clinically significant bleeding over clopidogrel, especially if they are used in patients with a lower bleeding risk, as usually occurs in routine clinical practice. However, this finding has to be interpreted considering that our cohort included STEMI patients who underwent reperfusion with primary PCI and who survived to the index episode, and thus it cannot be extrapolated to other contexts.
LimitationsSince we employed a clinical-administrative database to ascertain clinical outcomes during follow-up, the validity of our findings depends on the ability of the official health system registries to detect all episodes. However, the endpoints considered are hard outcomes and coverage of the official registries includes most the population, so a relevant underestimation of the outcome events rate is unlikely. This has been recently assessed in the general Spanish population for acute coronary syndrome.27 In addition, since the sources of information and definitions have remained the same since the beginning of the “Codi IAM” registry, the possible miscodification or underreporting should be also similar over time, thus the estimation of the change in exposures and outcomes over time should not be greatly affected. It has also to be noted that, although the sample is large enough to show important changes in treatment rates, it might be underpowered to detect slight changes over time in ischemic and hemorrhagic events for which changes are expected to be much lower. We studied a cohort of STEMI patients who underwent primary PCI and who survived the index episode for a better assessment of the effectiveness of other therapies for STEMI other than primary PCI. Therefore, our findings should not be extrapolated to other contexts. Finally, the pace of the incorporation of these high-level recommendation therapies into clinical practice cannot be extrapolated to other contexts. However, this lack of generalization should not affect the main focus of this work, which is the evaluation of the relationship between change in clinical practice over time and change in the prognosis of STEMI.
CONCLUSIONSThis large temporal trend outcomes analysis in patients with a revascularized STEMI has shown a strong increase between 2010 and 2017 in the use of recommended therapies with high-level evidence in the clinical setting, such as DES during primary PCI, ticagrelor and prasugrel at discharge, and an increase in the duration of DAPT after discharge. This change was followed by a small change in the rate at 2 years of follow-up of the composite of all-cause death, AMI, or stroke.
FUNDINGThis study was funded by a grant from Instituto de Salud Carlos III of the Spanish Health Ministry (Grant number: PI13/00399) and Marató de TV3 (Grand number: 430/U/2015).
AUTHORS’ CONTRIBUTIONSA. Ribera, M.T. Faixedas, A. Rosas, and I. Ferreira-González participated in the conception and design of the work. I. Ferreira-González, José A. Barrabés and Aida Ribera drafted the work. M.T. Faixedas, H. Tizón-Marcos, S. Rojas, C. Labata, M. Cárdenas, S. Homs, C. Tomás-Querol, J. García-Picart, Gerard Roura, Monica Masotti participated in data acquisition. J.R. Marsal, A. Ribera, J.I. Pijoan, J.A. Barrabés and I. Ferreira-González, participated in the data analysis. All authors participated in the interpretation of results, reviewed the draft critically and approved the final version.
CONFLICTS OF INTERESTNone.
This study was carried out using the linked anonymized data provided by the Agency for Health Quality and Assessment of Catalonia (AQuAS), within the framework of the PADRIS Program.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.10.011