Keywords
INTRODUCTION
Although the use of drug-eluting stents to treat coronary stenosis has dramatically decreased the need for new revascularization procedures compared to bare-metal stents,1-3 in-stent restenosis remains a clinical and biological problem.4 Furthermore, this problem is difficult to treat, especially when this occurs in drug-eluting stents; thus, careful stratification procedures are needed to identify the most vulnerable patients.
Recently, several biochemical markers have been carefully studied. These include high-sensitivity C-reactive protein (CRP) which is currently recognized as a strong predictor of cardiovascular events, including stroke, coronary artery disease, peripheral vascular disease, and sudden cardiac death.5,6
Histological studies have shown that percutaneous coronary intervention induces a significant inflammatory reaction in the vascular wall with local increases in biochemical markers, leukocyte infiltration, and neointimal hyperplasia.7-10 The role of inflammation in the pathogenesis of restenosis has been demonstrated in several studies.10,11 Thus, increased plasma CRP concentrations have been observed after stent implantation, peaking at 48 h and decreasing within 2 weeks.12,13 However, there are conflicting results regarding the association between CRP concentrations and the development of neointimal hyperplasia and in-stent restenosis.14-18 There is little research on the new drug-eluting stents, such as the ZoMaxx (Abbott) zotarolimus-eluting stent which was recently demonstrated as having a strong antiproliferative effect.19,20 Neither are there any published studies that associate CRP concentrations with neointimal hyperplasia after implanting stents of this type.
The aim of the present study was to assess the association between baseline CRP plasma concentrations and neointimal hyperplasia volume evaluated by intracoronary ultrasound (IVUS) at 4-month follow-up in patients treated with zotarolimus-eluting stents.
METHODS
The study included consecutive patients who underwent implantation with a ZoMaxx stent between February and June 2005 in our center.
The patients presented stable angina, unstable angina, or documented silent ischemia, with a new >50% diameter stenosis in a native coronary artery (measured by quantitative angiography), with a vessel diameter of >2.5 mm, and lesion length of <18 mm. Patients who presented acute myocardial infarction with left ventricle ejection fraction <30% or a >50% diameter left coronary trunk stenosis were excluded. Patients presenting kidney failure (baseline creatinine concentration >1.9 mg/dL), connective tissue disease, or those under treatment with corticosteroids were also excluded.
Quantitative angiography was conducted before and immediately after the procedure, and at 4-month follow-up. An IVUS study was conducted after stent implantation (baseline) and at 4-month follow-up.
Plasma high-sensitivity CRP (mg/L) concentrations were determined in the cardiac catheterization laboratory immediately before stent implantation, via a previously opened peripheral venous access route. C-reactive protein concentrations were determined by chemiluminescent substrate detection (IMMULITE® DPC, Los Angeles, CA, USA), with a cut-off value of 0.1 mg/L. Patients were divided into quartiles according to obtained and compared CRP concentrations.
Procedure
All the ZoMaxx stents used measured 3 mm in diameter by 18 mm in length. This new drug-eluting stent uses the TriMaxx stent (Abbott) as its platform, which is made up of 2 layers of stainless steel, a medial layer of tantalum, and with a strut thickness of 0.74 µm. The polymer is a biological compound of phosphorylcholine, nonreabsorbable, with an extra surface layer that allows controlled release of the drug within the first 30 days.19 Zotarolimus is a synthetic derivative of sirolimus with similar antiinflammatory and antiproliferative properties that has recently demonstrated efficacy in inhibiting neointimal hyperplasia in humans.19,20
The patients received aspirin (200 mg) and a loading dose of clopidogrel (300 mg) or ticlopidine (500 mg) at least 2 h or 48 h, respectively, before percutaneous coronary intervention. The permanent use of aspirin and of clopidogrel (75 mg/day) or ticlopidine (500 mg/day) for at least 3 months after the intervention was recommended.
All the interventions were performed according to current guidelines. All lesions were predilatated, using a balloon catheter less than or equal to 15 mm in length. Procedural success was defined as residual stenosis <30% with TIMI III (Thrombolysis In Myocardial Infarction) flow without major in-hospital complications.
Clinical follow-up was performed by coronary angiography and IVUS 4 months after the procedure. All patients were fully informed about the protocol and gave written consent. Quantitative analysis of the coronary angiography and IVUS results was performed by an experienced operator, blinded to the results and CRP concentrations.
Quantitative Coronary Angiography Analysis
Quantitative angiography was performed before and after stent implantation and at follow-up. Offline analysis was performed using a computer-assisted automated edge detection system (Cardiovascular Measurement Systems, version 5.1, Leiden, the Netherlands). The following were determined: late loss, defined as minimum lumen diameter (MLD) after stent implantation minus MLD at follow-up; net gain, defined as MLD at follow-up minus MLD before stent implantation; and binary restenosis, defined as a >50% diameter stenosis in the treated artery at follow-up (assessed by angiography).
Three-Dimensional Intracoronary Ultrasound
IVUS procedures were conducted after the intracoronary administration of 0.1-0.2 mg of nitroglycerin. Arterial segments were assessed using a 40 MHz transducer with an automatic pullback system at 0.5 mm/s (CVIS and Galaxy 2, Boston Scientific Corporation, Boston, Mass., USA). The acquired images were recorded on high-quality VHS video tape or CD/DVD for offline analysis.
The images were digitized for quantitative and qualitative analysis according to the American College of Cardiology's Clinical Expert Consensus Document.21 Three-dimensional reconstruction of the assessed segments was performed using an automated edge-detection system (Echoplaque, Indec Systems, Inc, Mountain View, CA, USA).
Cross-section measurements were taken at each 0.5-mm interval including the external elastic membrane (vessel), stent, lumen, and plaque (plaque plus media). Vessel, stent, lumen, and plaque volumes were calculated using Simpson's rule. Plaque volume (plaque behind the stent) was defined as "vessel volume minus stent volume." Intimal hyperplasia volume was calculated as "stent volume minus lumen volume." To eliminate the possible influence of vessel size, the percentage of stent obstruction was calculated as "intimal hyperplasia volume/stent volume x 100."
Statistical Analysis
Continuous variables are presented as mean and standard deviation. Discrete variables are presented as values and percentages.
One-way analysis of variance (one-way ANOVA) was used to assess differences between the 4 groups (quartiles) followed by a post-hoc Student-Newman-Keuls test when the ANOVA was positive. Discrete variables were compared using χ2 or Fisher test. Correlation (Pearson correlation coefficient) and linear regression analyses were performed to assess the relationship between C-reactive protein values and neointimal hyperplasia volumes.
A multiple linear regression model was constructed to evaluate any possible independent association between baseline variables (clinical, angiographic, or IVUS) and neointimal hyperplasia volume. A model with a maximum of 8 variables (1 for every 5 cases) was chosen, selecting those variables that previous studies had associated with neointimal hyperplasia and restenosis. The model was also adjusted for age and sex. The variables included: a) diabetes mellitus; b) CRP concentration; c) reference vessel diameter before implantation; d) stent deployment pressure; e) postimplantation MLD; f) postimplantation residual stenosis; g) postimplantation lumen volume; and h) postimplantation plaque volume. The analysis was performed based on the maximum likelihood model and by backward elimination of variables. The different models were compared using the partial F-test.
A 2-tailed P-value <.05 was considered significant for all analyses; the Statistical Package for Social Sciences (SPSS Inc., version 13.0) software was used.
RESULTS
Forty patients were successfully implanted with a ZoMaxx stent. Angiographic follow-up was performed in 37 patients (92.5%) and IVUS at 4 months.
The mean baseline CRP was 0.67 (0.96) mg/L. The population was divided into quartiles according to the value obtained. The mean CRP for the first quartile (1C) was 0.05 (0.03) mg/L; second quartile (2C), 0.23 (0.07) mg/L; third quartile (3C), 0.62 (0.2) mg/L; and fourth quartile (4C), 1.8 (1.3) mg/L (P<.001 for 1C, 2C, and 3C vs 4C).
There were no significant between-group differences in other baseline characteristics, including prevalence of diabetes, body mass index, cholesterol concentrations, and statin treatment (Table 1). The mean age was 58 (8) years, 55% of the patients were men, 40% presented diabetes mellitus, and 62% were undergoing statin treatment.
All lesions were predilatated. The mean length of the balloon was 13.05 (2.6) mm and mean diameter 2.5 (0.35) mm. The mean predilatation pressure was low (6.3 [2] atm), whereas the final stent deployment pressure was 12.98 (3.3) atm.
The baseline vessel, stent, plaque, and lumen volumes evaluated by IVUS were similar between groups, with a nonsignificant tendency toward larger volumes in 4C. At 4-month follow-up, a significant difference in vessel and plaque volume was observed between 1C and 2C versus 4C, due to a slight reduction in vessel and plaque volume in 1C and 2C, and to a slight increase in these volumes in 4C (Table 2). Similarly, post-hoc matched-pairs analyses (Student t test for matched samples) demonstrated that there was no significant difference in vessel volume and plaque volume after the procedure or at follow-up in each quartile (vessel volume: 1C: P=.06; 2C: P=.36; 3C: P=.56; 4C: P=.65. Plaque volume: 1C: P=.06; 2C: P =.65; 3C: P=.26; 4C: P=.2). The matched-pairs analysis demonstrated a significant difference in lumen volume between baseline and follow-up in the 3C and 4C (170 [42] µL vs 151 [43] µL, P=.02 for 3C; 204 [77] µL vs 189 [71] µL, P=.01 for 4C).
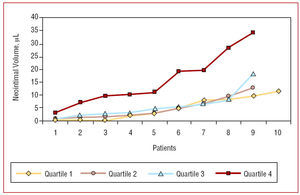
Intimal hyperplasia volume was 4.8 (4.2) µL for 1C, 4.9 (4) µL for 2C, 5.9 (4.3) µL for 3C, and 15.8 (10) µL for 4C (P<.01 for 1C, 2C, and 3C vs 4C, Figure 1). This difference was maintained when comparing the percentage of stent obstruction; these were 3.3% (3.8), 3.4% (2.9), 3.7% (2.8), and 8.0% (4), respectively (P<.01 for 1C, 2C, and 3C vs 4C, Table 2).
Figure 1. Distribution of neointimal hyperplasia volume (µL) in the different quartiles. There is a significant difference between quartile 4 and the other quartiles (ANOVA, P=.001)
The results of quantitative angiography before and after stent implantation were similar between groups and no significant differences were observed when the follow-up results were compared, such as in-stent late loss, the percentage of stenosis, and binary restenosis rate (Table 3).
Figure 2 shows a positive correlation between CRP concentrations and neointimal hyperplasia volume (r=0.64; 95% CI, 0.40-0.80; P<.0001). Similarly, Figure 3 shows a positive correlation between the CRP value and the percentage of stent obstruction (r=0.54; 95% CI, 0.26-0.73; P=.0005).
Figure 2. Correlation between C-reactive protein (CRP) (mg/L) and neointimal hyperplasia volume (µL). There is a significant positive correlation between the baseline CRP concentrations and neointimal hyperplasia volume at follow-up.
Figure 3. Correlation between C-reactive protein (CRP) (mg/L) and stent obstruction (%). There is a significant positive correlation between baseline CRP concentrations and the percentage of stent obstruction at follow-up.
There is an extreme value (outlier) in both figures representing a 55-year-old patient presenting the highest baseline CRP concentration in the series (5.2 mg/L). At follow-up, neointimal hyperplasia volume was 34 µl with 14.2% stent obstruction. By eliminating this extreme value, the correlation remains statistically significant, with a Pearson coefficient of 0.41 (P=.027) for CRP concentrations and neointimal hyperplasia volume, and 0.39 (P=.03) for CRP concentrations and percentage of stent obstruction.
The results of quantitative angiography showed there was no significant correlation between baseline CRP concentrations and in-stent late loss (r=0.19; P=.26).
Multiple linear regression analysis showed that the baseline variables independently associated with neointimal hyperplasia volume were as follows: postimplantation lumen volume measured by IVUS (β=0.06; P=.003), final stent deployment pressure (β=0.58; P<.02), and CRP value (β=3.9; P=.0001). The coefficient of determination of the model adjusted for age and sex was 0.67; P<.001 (Table 4).
Although not specified in the protocol, CRP concentrations were obtained at 4-month follow-up in 23 of the 40 patients, with a mean value of 0.45 (0.62) mg/L; post-hoc matched-pairs analysis indicated a significant reduction in CRP values at this time compared to their baseline values (P=.01).
DISCUSSION
Despite the importance of C-reactive protein as a predictor of cardiac events in patients with coronary artery disease, few studies have been designed with the aim of assessing the relationship between baseline CRP values and the extent of in-stent neointimal hyperplasia in patients treated with drug-eluting stents.
Thus, this is the first study to directly compare the extent of neointimal hyperplasia to CRP values measured immediately before the implantation of a zotarolimus-eluting stent by means of IVUS. A significant positive correlation was found between CRP concentrations and the extent of neointimal hyperplasia at 4-month follow-up.
Recently, the zotarolimus-eluting stent demonstrated satisfactory short-term and medium-term results.20,22,23 Meredith et al22 investigated the Endeavour® zotarolimus-eluting stent (Medtronic) observing an in-stent late loss of 0.33 (0.36) mm and stent obstruction of 4.5% (6.1) assessed by IVUS at 4-month follow-up. These data are consistent with the results obtained in the present analysis.
In-stent restenosis is mainly a process of excessive neointimal hyperplasia involving a cascade of events, including leukocyte infiltration on the vascular wall, and rapid smooth muscle cell migration and hyperplasia.9,24 This process begins as a response to acute injury due to vascular wall rupture associated with the percutaneous intervention, involving rapid progression within the first 4-8 months after the procedure.9
C-reactive protein is an acute phase reactant that has recently been directly studied as a risk marker for coronary artery disease.12,13,25 Recent studies have described a systemic inflammatory response determined by increased CRP concentrations after bare-metal12 and drug-elting13 coronary stenting, with a peak at 48 h after stent implantation and decreasing almost to baseline values 2 weeks after the procedure.12,13
Several studies recommend using CRP concentrations as an independent marker of events, including cardiac death, myocardial infarction, and restenosis after percutaneous intervention in coronary syndromes.26-32 However, the data supporting their use as a predictor of restenosis after coronary stenting have proven contradictory.33 Angioi et al34 reported a higher risk of angiographic restenosis in patients with increased baseline CRP. Walter et al15 showed that higher preprocedural CRP concentrations were related to angiographic restenosis in patients treated with bare-metal stents. Similar results were observed by Jeong et al35 in 272 patients undergoing balloon angioplasty and by Rahel et al36 in a study that included 600 patients. Recently, Dibra et al37 demonstrated that the size of the difference between postprocedural and baseline CRP values was associated with restenosis in patients treated with bare-metal stents. Similar to our results, Hong et al18 found a positive correlation between baseline CRP values and the extent of in-stent neointimal hyperplasia at 6-month follow-up assessed by IVUS in patients treated with bare-metal stents.
Regarding negative results, the GENERATION study,38 which included 483 patients, did not find an association between CRP concentrations and in-stent restenosis, although only 67% of patients underwent angiographic follow-up. The lack of association between preprocedural CRP concentrations and angiographic restenosis was also reported by Horne et al16 in 415 patients who underwent balloon angioplasty, atherectomy, or stenting. Recently, a similar finding was reported by Rittersma et al17 in 345 patients treated with bare-metal stents, all of whom underwent angiographic follow-up.
Despite these contradictory results, the probable role of CRP in restenosis was demonstrated by Ishikawa et al,39 who found a significant correlation between restenosis after directional coronary atherectomy and the degree of CRP immunoreactivity in extracted neointimal tissue. In this regard, Inoue et al40 recently reported that an increase in CRP concentrations in the lesion site after bare-metal stenting correlated with late loss.
In the present analysis we observed that the plasma CRP concentrations obtained before implanting zotarolimus-eluting stents was independently associated with the extent of neointimal hyperplasia at 4-month follow-up. Thus, patients with higher CRP concentrations had greater neointimal hyperplasia as assessed by IVUS.
At follow-up, a significant difference was found between groups in neointimal hyperplasia volume and the percentage of stent obstruction, and also a significant difference in vessel volume and plaque volume between 1C and 2C compared to 4C. It can be seen that this difference was due to a slight decrease in vessel volume and plaque volume in 1C and 2C compared to a slight increase in these volumes in 4C. We consider that the between-group differences observed in vessel volume and plaque volume at follow-up may be a finding of interest. Due to the lack of power in this analysis, we cannot attribute this difference to different vascular behavior between the groups due to vessel remodelling or plaque regression. Furthermore, the post-hoc matched-pairs analysis demonstrated that the difference in vessel volume and plaque volume between baseline and follow-up values was not significant in any of the quartiles.
In the multiple regression analysis, the CRP values, postimplantation lumen volume, and final stent deployment pressure were independent predictors of neointimal hyperplasia volume. We consider that the patients who presented active inflammation during stent implantation, as assessed by the baseline CRP level, developed quantitatively greater neointimal hyperplasia, whereas greater final stent deployment pressure, despite making it possible to reach a greater volume of the lumen, causes greater damage in the arterial wall and triggers a more marked proliferative response.
In contrast, no relationship was observed between CRP concentrations and in-stent late loss. Thus, although there is a correlation between in-stent late loss and neointimal hyperplasia volume in bare-metal stents and drug-eluting stents,41,42 IVUS generates a three-dimensional image that makes it possible to assess neointimal hyperplasia more homogenously over the entire length of the stent, leading to a more accurate quantitative measurement of the neointima. On the other hand, quantitative angiography, measuring MLD and late loss, determines in 2 dimensions the most critical stenotic areas which, 4 months after implanting a drug-eluting stent, do not reach substantial values. In relation to this apparent contradiction, IVUS would be a more sensitive tool for early quantitative evaluation of neointimal hyperplasia after implanting drug-eluting stents.
Limitations
The most important limitation of this study is the size of the sample and the fact that it does not have sufficient power to establish clinically relevant conclusions.
Given that the process of neointimal hyperplasia stabilizes between 4 and 8 months, evaluation at 4 months could overlook some neointimal growth.
Studies including more patients are required with longer follow-up time to determine the clinical value of these findings.
CONCLUSIONS
This study found that C-reactive protein concentrations measured immediately before zotarolimus-eluting stent implantation is an independent determinant of in-stent neointimal hyperplasia volume at 4-month follow-up.
These results could help to improve our understanding of the pathophysiology and prevention of neointimal hyperplasia after zotarolimus-eluting stent implantation.
ACKNOWLEDGEMENTS
We would like to acknowledge the intellectual and technical contributions of Dr Marcos Franchetti, Dr Guilherme Attizzani, Dr João G. Loures, Dr Edmilson Ishii, and Dr Arturo Quispe.
ABBREVIATIONS
C1: first quartile
C2: second quartile
C3: third quartile
C4: fourth quartile
CRP: C-reactive protein
IVUS: intracoronary ultrasound
MLD: minimum lumen diameter
See editorial on pages 903-6
This work won one of the awards for the best Ibero-American communication at the Congress of Cardiovascular Disease. Spanish Society of Cardiology, Malaga, October 2006.
Correspondence: Dr. A.A.C. Abizaid.
Avda. Dr. Dante Pazzanese 500, Ibirapuera (CEP 04012-909).
São Paulo. Brazil.
E-mail: aabizaid@uol.com.br
Received December 18, 2006.
Accepted for publication May 10, 2007.