Keywords
In recent years, implantable cardioverter defibrillators (ICD) have become the standard treatment for patients at high risk of sudden death due to ventricular arrhythmias.1 However, the fact that the ICD does not modify the arrhythmogenic substrate presents certain problems in its management during follow-up. Following implantation, many patients suffer recurrences of ventricular tachycardia (VT) with consequent device discharges.2-4 These episodes are infrequent in the majority of patients and antitachycardia pacing is normally effective in stopping them; consequently, defibrillator shocks are only required sporadically. In contrast, a small number of patients present frequent or incessant VT that require the use of multiple discharges, which diminish quality of life.5
Although radiofrequency ablation (RFA) has been proposed as a valid alternative for the management of VT,6-12 few results have been published regarding its long-term efficacy13,14 or its usefulness in patients with an ICD.15-17
The present study is a descriptive analysis of our experience with RFA of VT in patients with an ICD who presented electrical storm or incessant VT that could not be managed with other therapies. Our primary objective was to assess the capacity of RFA to interrupt the electrical storm and, consequently, control the clinical condition of these patients. In addition, we compared the initial results with the results of long-term follow-up of the procedure.
PATIENTS AND METHODS
Patients
Of the 441 patients in whom an ICD had been implanted in our hospital between January 1995 and August 2003, 11 presented electrical storm or incessant VT that required multiple defibrillator shocks. We defined electrical storm as the clinical condition in which the recurrent appearance of malignant arrhythmias requires 3 or more device discharges within 24 hours.2 The study included all patients with an ICD who received multiple defibrillator discharges due to presentation of electrical storm or incessant VT following failed reprogramming of the device and optimization of antiarrhythmic treatment. Patients who only presented isolated episodes of VT or in whom the situation could be controlled by reprogramming of the device or optimization of antiarrhythmic treatment were excluded.
Eighteen RFA procedures were performed in 11 male patients with a mean age of 67.64±5.87 years (Table 1). Ten of these patients had suffered myocardial infarct 15.50±5.08 years earlier (range, 4-17; 50th percentile [P50]=10). All had received an ICD 9.67 ±11.22 months previously (P50=17), in all cases indicated for sustained monomorphic VT. The mean ejection fraction was 29.11%±11.25%. Six patients were classified as New York Heart Association (NYHA) class I-II and none were class IV. Prior to ablation, 591.67±1020.34 episodes of VT were registered (range, 7-2604; P50=80) with 52.82±35.73 defibrillator discharges per patient (P50=40). Antiarrhythmic treatment (beta-blockers and/or amiodarone and/or sotalol) was always prescribed at the maximum tolerated dose.
Electrophysiological Study and Radiofrequency Ablation
After obtaining signed consent, an electrophysiological study was performed to confirm the diagnosis, characterize the spontaneous VT, and select the ablation site. Using a percutaneous approach through the femoral vein, bipolar electrode catheters were introduced into the right ventricular apex or outflow tract in an attempt to induce ventricular arrhythmias. A programmable stimulator (Biotronik UHS 20, Biotronik GmbH, Berlin, Germany) was used to apply up to 3 extrastimuli during sinus rhythm and on paced cycle lengths of 600 ms and 400 ms.
An ablation catheter was then introduced via the femoral artery and guided to the site of interest, generally the left ventricle. Conventional mapping studies were undertaken that included the following: a) activation mapping of VT; b) detection of prolonged, fractionated middiastolic potentials and early presystolic activity; and c) entrainment of tachycardia. The following electrophysiological criteria were used to select the ablation site: a) middiastolic and presystolic potentials; b) entrainment of VT with concealed fusion and the return cycle length equal to the VT cycle length; and c) presystolic potentials with an activation time to the QRS identical to the stimulus-to-QRS interval.
Although these patients are known to present multiple VT, its inducibility does not imply that it represents a problem for the patient. Consequently, only VT identified as causing electrical storm was treated, the suppression of this condition being the main objective of the study. Radiofrequency was applied using a system that allowed temperature control (up to 70°C). In 1 patient, an electrode catheter with an irrigated tip was used following the failure of conventional catheters. In all procedures, fluoroscopic control was employed, endocardial potentials were monitored, and the ICD was disconnected.
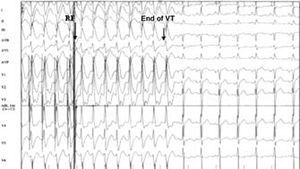
When the ablation was completed, programmed ventricular stimulation was performed in an attempt to reinduce tachycardia and assess electrophysiological outcome. Clinical success was considered as the interruption of electrical storm or incessant VT, while electrophysiological success was defined as the cessation of spontaneous VT (Figure 1) along with an inability to reinduce it. Induction of a treated VT (sustained or nonsustained) following treatment was considered as failure. Given that only spontaneous VT was treated, the induction of other untreated VTs did not influence the definition of the result.
Figure 1. Electrogram showing an example of an electrophysiologically successful ablation. Arrows indicate the moment of radiofrequency application and the end of the tachycardia 8 complexes later when the sinus rhythm is recovered.
Follow-up
Following hospital discharge, antiarrhythmic medication was suspended (except beta-blockers), particularly when electrophysiological success had been achieved. Follow-up appointments were programmed for every 6 months and in the event of discharge of the ICD. These appointments consisted of interrogation of the device to detect both untreated (nonsustained or slow VT) and treated episodes. Check-ups required for the underlying heart disease were not recorded.
The electrophysiological study was only repeated in very specific circumstances that could indicate a requirement for repeat RFA, such as initial clinical failure or the reappearance of uncontrollable VT. A 12-lead electrocardiogram was only available in cases in which recurrences of arrhythmia occurred in hospital; in all other cases recordings were obtained from the memory of the ICD. Recorded VT episodes were designated as recurrences of the ablated VT or as different VT events according to the cycle length (a variation of ±20 ms was allowed) and/or the similarity of the stored electrogram.
Statistical Analysis
Statistical analysis was performed using the SPSS software package version 9.0 (SPSS Inc, 1999). Continuous variables are expressed as means±SD and range, and in some cases, the 50th percentile (P50) is also shown. Means were compared using the Student's t test for paired data. Kaplan-Meier curves were used to calculate the cumulative probability of remaining free of recurrence or death by any cause. Statistical significance was established at P<.05.
RESULTS
Immediate Results
At the time of the first procedure, 6 patients were suffering from electrical storm and 5 from incessant VT. In all cases, a surface electrocardiogram revealed a single tachycardia responsible for the condition; the mean cycle length was 383.60±36.52 ms with a range of 330-440 ms.
In the baseline electrophysiological study, the induced VT was assumed to be the same as the spontaneous VT if it had the same morphology and a similar or slightly longer cycle length (caused by the antiarrhythmic drugs). Data was only collected in relation to this VT, which was the target VT. The cycle length was 407±42.18 ms (range, 340-500 ms). Only the clinical VT was treated in 10 of the patients. However, in 1 patient this VT was easily converted into 3 rapid and poorly tolerated VTs and it was decided to treat them all in the same session.
A mean of 1.63±0.69 procedures were performed per patient (range, 1-3); 2 attempts were required in 5 patients and 3 sessions were required in 1 patient. The mean number of radiofrequency applications was 16.73±12.07 and the mean fluoroscopy time was 49.14±26.04 min per patient. An electrophysiological success rate of 72.73% (n=8) was obtained following the first procedure. An irrigated-tip catheter was used in attempts 2 and 3 for patient 7 due to failure of the conventional catheter. In other cases, the induction of nonsustained VT following RFA was considered as initial electrophysiological failure. However, attempts with different catheters were rejected since in all cases the main objective of treating the electrical storm or incessant VT was achieved.
In general, the procedure was well tolerated. Only 2 cases of transient arterial hypotension were produced, representing a rate of complications of less than 11%. No mortality or major complications were associated with RFA; neither were there any cases of ICD dysfunction. The data associated with the procedure are shown in Table 2. Upon discharge, antiarrhythmic medication that had been prescribed to manage the recurrent VT was stopped. Treatment with beta-blockers was maintained due to prior infarct and cardiac failure. It was only necessary to reinitiate treatment with amiodarone, following recurrence, in patient 8, who subsequently remained asymptomatic and free of new arrhythmias.
Follow-up
Alter a mean follow-up period of 39.10±24.70 months from the last, definitive procedure in each patient, the mean number of cardioversion shocks per patient was reduced from 52.82±35.73 with a P50 of 40 to 0.64±1.03 with P50 of 0 (Figure 2; P<.001), irrespective of electrophysiological success. Six patients (54.50%) did not receive any cardioversion shocks, despite the fact that in 2 of them RFA was considered electrophysiologically ineffective; the remaining 5 patients (45.50%) only received occasional shocks (Table 2).
Figure 2. Evolution of the number of cardioversion shocks delivered in each patient; the baseline situation is compared with the situation following the definitive procedure in each patient. Irrespective of the electrophysiological result, the number of shocks was reduced in all patients and disappeared in many of them.
Nine patients presented subsequent episodes of VT (Table 2). The electrograms of 5 of these recurrences were similar to those of the treated VT; this VT had been suppressed following the first RFA in 4 patients and nonsustained VT had been induced in 1 patient. The recurrences were slower and, in general, nonsustained; the majority of the episodes were easily controlled by burst pacing and drug treatment. The cardioversion shocks were highly sporadic.
When these results were analyzed chronologically, the probability of remaining free of recurrences with an electrogram the same as the treated VT, estimated using Kaplan-Meier curves, was 81%, 81%, and 67% at 12 months, 24 months, and 36 months, respectively (Figure 3). However, the probability calculated for any VT was much lower: 44%, 33%, and 22% at 12 months, 24 months, and 36 months, respectively. After 39 months, no new incidences of arrhythmia were recorded.
Figure 3. Kaplan-Meier curves showing the probability of remaining free of recurrences of the ablated tachycardia (A) or any tachycardia (B) over a follow-up period of up to 69 months. VT indicates ventricular tachycardia.
The procedure had to be repeated in 6 patients (2 due to initial failure, 3 due to recurrence of a similar VT, and 1 due to a different VT). If we consider only the final session in each patient, the electrophysiological success rate was 72.73%.
Two patients died subsequently due to progression of heart failure; the ablation had been effective in both patients. In 1 of these patients (patient 4) a new electrical storm was recorded at 3 months, due to a different VT that could not be induced previously. The overall survival was 81.82% (Figure 4). Although standardized quality of life tests were not performed, the 9 surviving patients expressed a significant improvement, a finding that is consistent with the reduction of symptoms and, in particular, ICD discharges.
Figure 4. Kaplan-Meier curve of survival during the study.
DISCUSSION
Use of the ICD has significantly altered the prognosis of patients at risk of sudden death due to ventricular arrhythmias.1,18 However, this device stops the VT without preventing new ones appearing and 30% to 70% of these patients require concomitant antiarrhythmic therapy.19,20 Nevertheless, 68% of patients present recurrences of VT5,15-17 that in many cases is due to the coexistence of a myocardial infarct scar and left ventricular dysfunction that creates complex reentry circuits with multiple possibilities of VT. In 3% to 5% of patients, electrical storm or incessant VT is presented in follow-up and characteristically appears some time after infarct.2-4
Furthermore, alternative attempts for managing these conditions yielded disappointing results. ICD reprogramming and adjustment of medical treatment are insufficient in many cases of VT,19,20 especially in those that are highly recurrent.2-4 Furthermore, revascularization is not always feasible or effective.21 Currently, RFA is a valid alternative for the treatment of VT6-10,12-14 and its increased use is favored by the improved results obtained due to the technological innovations introduced in the mapping and ablation procedures.22-28
Success of RFA is accepted as the lack of inducibility of the VT. However, there is insufficient data available regarding the subsequent clinical evolution according to the result of the procedure. Also, the rate of recurrence in patients in whom the ablation is considered an electrophysiological failure is unknown. The electrophysiological results of RFA can be disheartening, since the theoretical objective of noninducibility of the spontaneous VT is not always achieved. Nevertheless, the clinical response can be favorable even in cases in which inducibility has not been suppressed,16 as shown by the disappearance of sustained and/or symptomatic arrhythmias. Independently of the abolition of VT, the natural history of these patients is altered substantially and they only present isolated events that usually consist of very rapid VTs that are assumed to be different and are controlled with isolated cardioversion shocks. This repercussion in the follow-up is probably due to modification of the substrate and the electrophysiological characteristics of the circuit by the radiofrequency, an effect that facilitates pharmacological control of the episodes (impossible prior to the intervention) and allows cessation or reduction of the number of ICD discharges, leading to improved quality of life for the patients.8,29
This study, based on a small but representative group, included a high percentage of ischemic patients with a highly depressed ejection fraction, whose characteristics differed markedly from patients with VT and conserved or moderately depressed ventricular contractility. In such a population it would be reasonable to expect limited success.12 Nevertheless, we achieved an initial electrophysiological success rate of 72.73% and an initial clinical success rate of 100%, with persistence of clinical results of more than 3 years in many patients. Although the rate of recurrence of arrhythmias in the long term may appear disappointing, these episodes were largely isolated. Two factors must be taken into account in order to understand the apparent discrepancy between the recurrence of arrhythmias and the clinical result. Firstly, since the main objective was the interruption of electrical storm and not all ventricular arrhythmias, only the causative VT was treated. Secondly, as mentioned, the inherent characteristics of the patients meant that few could be expected to be exempt from new ventricular arrhythmias and ablation would not normally be curative in this broader sense. Consequently, RFA is a complementary or, in some cases, palliative tool that is nevertheless necessary to complement the efficacy of the ICD and resolve dramatic situations in which the device and/or antiarrhythmic therapy are insufficient.
Another important factor is the period of observation of the patients. The majority of studies similar to this one have been based on short-term follow-up.15-17 Thus, Williams et al15 published the cases of 6 patients followed for between 5 and 19 months, Strickberger et al16 analyzed 21 patients over a period of 11.8±10 months, and Gonska et al13 employed a mean follow-up of 24 months.13 Our results show that the clinical benefit is maintained over even longer periods (mean, 39.1 months), irrespective of the electrophysiological outcome achieved. Not all of the VTs present were abolished, since these others do not always become a problem for the patient. Furthermore, following the procedure, we even stopped antiarrhythmic therapy (indicated in an attempt to control electrical storm) without it leading to recurrence of arrhythmia. Initially, only beta-blockers were maintained, for obvious reasons, and it was necessary to subsequently reintroduce amiodarone in only 1 patient. Nevertheless, the reduction in the number of ICD discharges was significant in all cases, independently of the total suppression of inducibility following the procedure (Figure 2). We conclude from our findings that clinical evolution and electrophysiological outcome are not necessarily parallel and that, in our patients, the clinical situation rather than the number of persistent VTs should decide whether or not antiarrhythmic therapy is maintained.
Classification of recurrences as the same as or different to the treated VT is difficult. We employed morphological and cycle length criteria and found that VTs different to the initial one were found more frequently and earlier. Nevertheless, since it is possible for the radiofrequency to modify the circuit (and, therefore, the morphology of the VT) we considered it pertinent to include all episodes together in Figure 3B.
It is worth bearing in mind the 2 most important limitations of this study. Firstly, since the patient series was small, we were unable to identify factors associated with success, failure, and/or recurrence. Secondly, the absence of a control group prevented definition of the benefit that was directly attributable to the ablation. Nevertheless, our results highlight the importance of offering this procedure in a growing number of patients for whom there is no lack of resources, such as implantation of expensive ICD devices, that reduce mortality but whose quality of life can be seriously diminished.
We conclude that RFA constitutes a good therapeutic option for patients with ICDs who present multiple episodes of VT with the appropriate corresponding discharges, and that it is capable of effectively interrupting the desperate situations of electrical storm or incessant VT. The clinical benefit of RFA is maintained in the long term in almost all patients and allows a significant reduction in the number of cardioversion shocks in all of them. However, given that ablation does not prevent the reappearance of the malignant arrhythmias to which these patients are particular susceptible, it should be considered complementary to the ICD rather than a substitute for it.
Correspondence: Dra. A.M. Montijano Cabrera.
Avda. Dr. Fleming, 4, 2.o 3.a. 14004 Córdoba. España.
E-mail: amontijano@medynet.com
Received January 7, 2004.
Accepted for publication February 17, 2005.