Liver fibrosis is present in nonalcoholic liver disease (NAFLD) and both precede liver failure. Subclinical forms of liver fibrosis might increase the risk of cardiovascular events. The objective of this study was to describe the prognostic value of the FIB-4 index on in-hospital mortality and postdischarge outcomes in patients with acute coronary syndrome (ACS).
MethodsRetrospective study including all consecutive patients admitted for ACS between 2009 and 2019. According to the FIB-4 index, patients were categorized as <1.30, 1.30-2.67 or> 2.67. Heart failure (HF) and major bleeding (MB) were assessed taking all-cause mortality as a competing event and subhazard ratios (sHR) are presented. Recurrent events were evaluated by the incidence rate ratio (IRR).
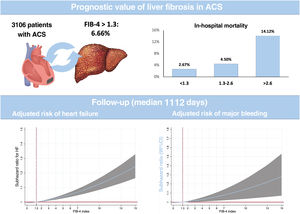
ResultsWe included 3106 patients and 6.66% had a FIB-4 index ≥ 1.3. A multivariate analysis verified a higher risk of in-hospital mortality associated with the FIB-4 index (OR, 1.24; P=.016). Patients with a FIB-4 index> 2.67 had a 2-fold higher in-hospital mortality risk (OR, 2.35; P=.038). After discharge (median follow-up 1112 days), the FIB-4 index had no prognostic value for mortality. In contrast, patients with FIB-4 index ≥ 1.3 had a higher risk of first (sHR, 1.61; P=.04) or recurrent (IRR, 1.70; P=.001) HF readmission. Similarly, FIB-4 index ≥ 1.30 was associated with a higher MB risk (sHR, 1.62; P=.030).
ConclusionsThe assessment of liver fibrosis by the FIB-4 index identifies ACS patients not only at higher risk of in-hospital mortality but also at higher risk of HF and MB after discharge.
Keywords
Liver function abnormalities are common in patients with coronary heart disease due to the high prevalence of obesity, diabetes, and heart failure.1,2 Nonalcoholic fatty liver disease (NAFLD) and liver fibrosis are known to precede cirrhosis and liver dysfunction.1,2
The FIB-4 (Fibrosis Index Based on 4 Factors) index is a feasible tool for the assessment of liver fibrosis3,4 and its diagnostic accuracy for predicting advanced fibrosis in patients with biopsy-proven NAFLD is similar to that of magnetic resonance.5 Liver cirrhosis with portal hypertension is included as major criteria for defining patients at high risk of bleeding6 but mild or moderate liver abnormalities might increase the risk of major bleeding (MB)4 and, even more, heart failure,7,8 major cardiovascular events (MACE) or mortality.9 Although a portion of the risk of cardiovascular complications from liver fibrosis or NAFLD is attributable to these comorbidities, diagnosis of NAFLD is associated with a greater risk than the sum of risk factors related to its incidence.4
Under these premises, we investigated the prognostic value of liver fibrosis, assessed by the FIB4 index, in a cohort of patients admitted for an acute coronary syndrome (ACS).
METHODSWe performed a retrospective study of all consecutive patients admitted for ACS to the Cardiology Department of Hospital de San Juan (Alicante, Spain) between 2009 and 2019. A total of 3174 patients were admitted and after exclusion of 8 patients who were HIV seropositive and 29 with known hepatitis, 3106 patients were analyzed. ACS was defined by the presence of typical clinical symptoms of chest pain and electrocardiographic changes indicative of myocardial ischemia/lesion and/or elevation of serum markers of myocardial damage.10 Liver fibrosis was estimated by the FIB4 index,1 which is an algorithm based on platelet count, age, alanine transaminase (ALT) and aspartate transaminase (AST): age (years) x AST [U/L]/(platelet count [10^3/μL] ×ALT [U/L]). According to the recommendations of the Nonalcoholic Steatohepatitis Clinical Research Network scoring system, patients were categorized as 1.30, 1.30 to 2.67, or> 2.67.1,9,11
Patients were classified as ST-elevation myocardial infarction (STEMI) and non–ST-elevation acute coronary syndrome (NSTEACS) according to electrocardiographic findings. Risk factors, medical history, treatments, complementary tests, and principal diagnosis at discharge were registered from all patients by trained medical staff. The diagnostic and therapeutic ACS protocols of the center include blood sample determinations in the emergency department and the first fasting state after hospital admission. AST is usually available since the first blood test in the emergency tests and ALT in the first 24hours. The first determination of each patient was collected.
DefinitionsAccording to the 2019 Academic Research Consortium for High Bleeding Risk (ARC-HBR) definition,6 patients were defined according to the ARC-HBR consensus if they met at least 1 major or 2 minor criteria. Glomerular filtration rate was estimated using the Modification of Diet on Renal Disease equation.12 Comorbidities were assessed by the Charlson index, adapted for patients with cardiovascular disease,13 and patients with Charlson score> 4 qualified for high-comorbidity burden. The completeness of revascularization was prospectively determined after the revascularization procedure based on the intended “equivalent anatomic” revascularization before revascularization based on segment numbering of vessels with a diameter> 1.5mm.14
Endpoints and follow-upThe primary endpoints were all-cause and cardiovascular mortality, first heart failure (HF) hospitalization, and first major bleeding (MB); secondary endpoints were the cumulative incidence of subsequent HF and MB. Diagnosis of HF was codified according to medical reports, signed by the medical staff of each institution, and was mainly based on the diagnostic criteria of clinical guidelines.15 MB were collected according to the Thrombolysis and Myocardial Infarction (TIMI) bleeding scale for noncoronary artery bypass grafting-related major bleeding and those fitting definitions 3 or 5 of the Bleeding Academic Research Consortium.16 The study protocol was approved by the ethics committee of the coordinating hospital and informed consent was obtained from all patients.
The postdischarge follow-up of patients had a well-established protocol and was made by telephone calls and review of electronic medical reports and institutional databases. Vital status was determined by telephone calls in the absence of medical reports. The study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.
Statistical analysesQuantitative variables are presented as mean±SD, and differences were assessed by the Student t and chi-square tests. Qualitative variables are presented as percentages, and differences were analyzed by ANOVA. As expected, age, glomerular filtration rate, GRACE score, the PRECISE-DAPT score and the FIB-4 index obtained positive colinearity results and were modeled in categorical variables; nonetheless, none obtained positive results in the multivariate analyses. Variables associated with in-hospital mortality were assessed by logistic binary regression. The calibration of the model was assessed by the Hosmer-Lemeshow test and diagnostic accuracy by the area under the curve of the probability of the diagnosis; the overall assessment was represented in the calibration belt,17 which allows the ranges of risk to be spotted where there is a significant deviation from the ideal calibration, and the direction of the deviation to be indicated.
Survival analyses were performed by Cox-regression models, after verification of the proportional risk assumption by the Schoenfeld residuals test. Prior to entry into regression models, the FIB-4 score values were expanded using fractional polynomials so as not to assume linearity of effect.
The risk estimates for postdischarge HF or MB could be affected by patients’ survival status. Therefore, the usual techniques for time-to-event analysis would provide biased or un-interpretable results due to competing risks, and the Kaplan-Meier estimation would overestimate the HF incidence. To avoid such effects, we applied the model introduced by Fine and Gray18 to test the competing events. The incidence of HF and MB is presented in cumulative incidence function graphs and the results of the multivariate analysis are presented as subhazard ratios (sHR) and corresponding 95% confidence intervals (95%CI). Harrell's c-statistic test was used to assess the discrimination of the model. Calibration was tested by the Gronnesby and Borgan test. Patients lost to follow-up were categorized as missing, as well as those who lacked any of the main variables for the analyses, although these accounted for less than 5%. For the assessment of the reclassification of the actual risk of MB of the FIB-4, taking the PRECISE-DAPT as the gold-standard, we assessed the reclassification rate (%), the net reclassification improvement and the integrated discrimination improvement.19 For their calculation, no censored data were allowed.
Recurrent hospitalizations were evaluated by determining the incidence rate ratio (IRR). Because an increase in HF hospitalizations is associated with an increased risk of subsequent death, it has been suggested that any analysis of recurrent admissions should also account for death as a terminal event. Thus, coefficients from this method were estimated by accounting for the positive correlation between the recurrent outcome and death as a terminal event by linking the 2 simultaneous equations (rehospitalization count and death) with shared frailty, using the bivcnto STATA command.20 Thus, within the same model, we obtained estimates of risk for both endpoints. Covariate selection was performed based on previous medical knowledge. The covariates included in the final predictive model for the endpoints were age, sex, diabetes, revascularization, previous HF, left ventricular ejection fraction, hemoglobin, atrial fibrillation, and medical treatments at discharge.
Statistical difference was accepted at P <.05. All analyses were performed using STATA 14.3 (StataCorp, 2009, Stata Statistical Software: Release 14, StataCorp LP, United States).
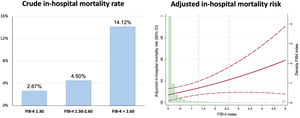
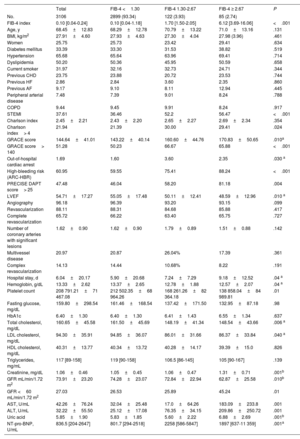
RESULTSThe clinical features of the cohort are presented in table 1. Among the 3106 patients, the median FIB-4 index was 0.10 (0.04-0.24) and 6.66% had a FIB-4 index ≥ 1.30. Interestingly, no significant differences were observed in most clinical features but patients with a FIB-4 index> 2.67 were more frequently admitted as STEMI, had more out-of-hospital cardiac arrests, and had higher GRACE score. No differences in coronary lesions or revascularization were observed. In contrast, significant differences were observed in biochemical determinations (table 1 Patients with a FIB-4 index <1.30 had higher hemoglobin, total cholesterol, and low-density lipoprotein cholesterol but the lowest NT-pro-BNP levels. Patients with a FIB-4 index> 2.67 had the lowest glomerular filtration rate and highest levels of uric acid. A significant trend (P=0021) to higher FIB-4 index values was found according to higher Killip class but FIB-4 was only significantly higher in patients with Killip-IV compared with Killip-I (1.80 [6.03] vs 0.56 [5.79]; P=.031). Among the 1633 categorized as non-high bleeding risk, 67 (4.1%) had a FIB-4 index> 1.30.
Clinical features of the cohort according to the FIB4 index
| Total | FIB-4 <1.30 | FIB-4 1.30-2.67 | FIB-4 ≥ 2.67 | P | |
|---|---|---|---|---|---|
| No. | 3106 | 2899 (93.34) | 122 (3.93) | 85 (2.74) | |
| FIB-4 index | 0.10 [0.04-0.24] | 0.10 [0.04-1.18] | 1.70 [1.50-2.05] | 6.12 [3.69-16.06] | <.001 |
| Age, y | 68.45±12.83 | 68.29±12.78 | 70.79±13.22 | 71.0±13.16 | .131 |
| BMI, kg/m2 | 27.91±4.60 | 27.93±4.63 | 27.30±4.04 | 27.98 (3.96) | .461 |
| Women | 25.75 | 25.73 | 23.42 | 29.41 | .634 |
| Diabetes mellitus | 33.39 | 33.30 | 31.53 | 38.82 | .519 |
| Hypertension | 65.68 | 65.64 | 63.96 | 69.41 | .714 |
| Dyslipidemia | 50.20 | 50.36 | 45.95 | 50.59 | .658 |
| Current smoker | 31.97 | 32.16 | 32.73 | 24.71 | .344 |
| Previous CHD | 23.75 | 23.88 | 20.72 | 23.53 | .744 |
| Previous HF | 2.86 | 2.84 | 3.60 | 2.35 | .860 |
| Previous AF | 9.17 | 9.10 | 8.11 | 12.94 | .445 |
| Peripheral arterial disease | 7.48 | 7.39 | 9.01 | 8.24 | .788 |
| COPD | 9.44 | 9.45 | 9.91 | 8.24 | .917 |
| STEMI | 37.61 | 36.46 | 52.2 | 56.47 | <.001 |
| Charlson index | 2.45±2.21 | 2.43±2.20 | 2.65±2.27 | 2.69±2.34 | .354 |
| Charlson index> 4 | 21.94 | 21.39 | 30.00 | 29.41 | .024 |
| GRACE score | 144.64±41.01 | 143.22±40.14 | 160.60±44.76 | 170.83±50.65 | .010a |
| GRACE score> 140 | 51.28 | 50.23 | 66.67 | 65.88 | <.001 |
| Out-of-hospital cardiac arrest | 1.69 | 1.60 | 3.60 | 2.35 | .030 a |
| High-bleeding risk (ARC-HBR) | 60.95 | 59.55 | 75.41 | 88.24 | <.001 |
| PRECISE DAPT score> 25 | 47.48 | 46.04 | 58.20 | 81.18 | .004 |
| LVEF | 54.71±17.27 | 55.05±17.48 | 50.11±12.41 | 48.59±12.96 | .010 a |
| Angiography | 96.18 | 96.39 | 93.20 | 93.15 | .099 |
| Revascularization | 88.11 | 88.31 | 84.68 | 85.88 | .417 |
| Complete revascularization | 65.72 | 66.22 | 63.40 | 65.75 | .727 |
| Number of coronary arteries with significant lesions | 1.62±0.90 | 1.62±0.90 | 1.79±0.89 | 1.51±0.88 | .142 |
| Multivessel disease | 20.97 | 20.87 | 26.04% | 17.39 | .361 |
| Complex revascularization | 14.13 | 14.44 | 10.68% | 8.22 | .191 |
| Hospital stay, d | 6.04±20.17 | 5.90±20.68 | 7.24±7.29 | 9.18±12.52 | .04 a |
| Hemoglobin, g/dL | 13.33±2.62 | 13.37±2.65 | 12.78±1.88 | 12.57±2.07 | .04 a |
| Platelet count | 208 791.21±71 467.08 | 212 502.35±68 964.26 | 168 261.26±82 364.18 | 138 858.04±84 989.81 | .01 |
| Fasting glucose, mg/dL | 159.80±298.54 | 161.46±168.54 | 137.42±171.50 | 132.95±87.18 | .98 |
| HbA1c | 6.40±1.30 | 6.40±1.30 | 6.41±1.43 | 6.55±1.34 | .637 |
| Total cholesterol, mg/dL | 160.65±45.58 | 161.50±45.69 | 148.19±41.34 | 148.54±43.66 | .006 a |
| LDL cholesterol, mg/dL | 94.30±35.91 | 94.85±36.07 | 86.01±31.66 | 86.37±33.84 | .040 a |
| HDL cholesterol, mg/dL | 40.31±13.77 | 40.34±13.72 | 40.28±14.17 | 39.39±15.0 | .826 |
| Triglycerides, mg/mL | 117 [89-158] | 119 [90-158] | 106.5 [86-145] | 105 [90-167] | .139 |
| Creatinine, mg/dL | 1.06±0.46 | 1.05±0.45 | 1.06±0.47 | 1.31±0.71 | .001b |
| GFR mL/min/1.72 m2 | 73.91±23.20 | 74.28±23.07 | 72.84±22.94 | 62.87±25.58 | .010b |
| GFR <60 mL/min/1.72 m2 | 27.03 | 26.53 | 25.89 | 45.24 | .01 |
| AST, U/mL | 42.26±76.24 | 32.04±25.48 | 17.0±64.26 | 183.09±233.8 | .001 |
| ALT, U/mL | 32.22±55.50 | 25.12±17.08 | 76.35±34.15 | 209.86±250.72 | .001 |
| Uric acid | 5.85±1.90 | 5.83±1.85 | 5.60±2.22 | 6.88±2.69 | .001b |
| NT-pro-BNP, U/mL | 836.5 [204-2647] | 801.7 [294-2518] | 2258 [586-5847] | 1897 [637-11 359] | .001a |
ALT, alanine transaminase; AST, aspartate transaminase; COPD, chronic obstructive pulmonary disease; HDL, high-density lipoprotein cholesterol; GFR, glomerular filtration rate; LDL, low-density lipoprotein cholesterol; LVEF, left ventricle ejection fraction; STEMI, ST-elevation myocardial infarction.
Values expressed as percentages, No. (%), mean±standard deviation, or median [interquartile range].
Patients with FIB-4 index <1.30 had shorter hospital stay and higher left ventricular ejection fraction at discharge. The in-hospital mortality rate was 3.06% (92 patients). As shown in figure 1, the crude in-hospital mortality rate was higher in each category of FIB-4 index. The multivariate analysis, adjusted by age, sex, diabetes, previous coronary heart disease, hemoglobin, revascularization, and GRACE score, showed a significant association between in-hospital mortality and FIB-4 index and in-hospital mortality risk (OR, 1.24; 95%CI, 1.04-1.47; P=.016). When analyzed by categories, patients with a FIB-4 index> 2.67 had a 2-fold higher in-hospital mortality risk (OR, 2.35; 95%CI, 1.045-5.278; P=.038) but no significant increase was noted for patients categorized as 1.30-2.67 (OR, 0.93; 95%CI, 0.33-2.64; P=.891). The model was well calibrated (P=.97) and had fair discriminatory ability (figure 1 of the supplementary data).
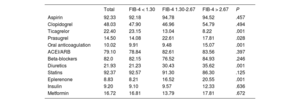
Postdischarge outcomesThe medical treatments recommended at discharge are presented in table 2. Patients with a FIB-4 index> 2.67 less frequently received ticagrelor or prasugrel, but more frequently received anticoagulation, eplerenone and diuretics. Postdischarge follow-up was available in 96% of the cohort with a median follow-up of 1112 [interquartile range 633-1796] days. During that time, 464 (15.8%) patients died and 308 (10.52%) deaths were attributable to cardiovascular causes. Patients categorized with FIB-4 index> 1.30 had higher crude mortality rates (figure 1 of the supplementary data); nonetheless, after adjustment by age, sex, diabetes, revascularization, left ventricular ejection fraction, GRACE score and medical treatments, the FIB-4 index had no prognostic value.
Medical treatment at discharge according to the FIB-4 index
| Total | FIB-4 < 1.30 | FIB-4 1.30-2.67 | FIB-4 > 2.67 | P | |
|---|---|---|---|---|---|
| Aspirin | 92.33 | 92.18 | 94.78 | 94.52 | .457 |
| Clopidogrel | 48.03 | 47.90 | 46.96 | 54.79 | .494 |
| Ticagrelor | 22.40 | 23.15 | 13.04 | 8.22 | .001 |
| Prasugrel | 14.50 | 14.08 | 22.61 | 17.81 | .028 |
| Oral anticoagulation | 10.02 | 9.91 | 9.48 | 15.07 | .001 |
| ACEI/ARB | 79.10 | 78.84 | 82.61 | 83.56 | .397 |
| Beta-blockers | 82.0 | 82.15 | 76.52 | 84.93 | .246 |
| Diuretics | 21.93 | 21.23 | 30.43 | 35.62 | .001 |
| Statins | 92.37 | 92.57 | 91.30 | 86.30 | .125 |
| Eplerenone | 8.83 | 8.21 | 16.52 | 20.55 | .001 |
| Insulin | 9.20 | 9.10 | 9.57 | 12.33 | .636 |
| Metformin | 16.72 | 16.81 | 13.79 | 17.81 | .672 |
Values expressed as percentages. ACEI, angiotensin-converter enzyme inhibitors; ARB, angiontensin receptor blockers.
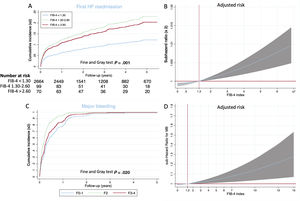
A total of 328 (10.41%) patients had at least 1 hospital readmission for HF and 179 (5.28%) had 2 or more HF rehospitalizations. As shown in figure 2A, patients with a FIB-4 index <1.30 had a significantly lower risk of HF readmission and, after adjustment by age, sex, diabetes, revascularization, previous HF, left ventricular ejection fraction, hemoglobin, atrial fibrillation, glomerular filtration rate and NT-pro-BNP and medical treatment, a continuous risk was observed according to the FIB-4 index (figure 2B). Patients with a FIB-4 index> 1.30 had a higher risk of first HF readmission (sHR, 1.61; 95%CI, 1.02-2.44; P=.04).
The highest rate of recurrent HF hospitalizations was observed in patients with a FIB-4 index> 2.67 (10.33 per 100 patients), followed by patients with a FIB-4 index 1.30-2.67 (4.87 per 100 patients). Differences remained after adjustment by age, sex, diabetes, revascularization, previous HF, hemoglobin, atrial fibrillation, and medical treatment. A FIB-4 index> 2.67 was independently associated with a higher risk of recurrent HF readmissions (IRR, 1.70; 95%CI, 1.25-2.30; P=.001).
Major bleedingThe postdischarge MB incidence was 6.19% (195 patients). As shown in figure 2C, patients with a FIB-4 index> 1-30 had significantly higher crude rates of MB. After adjustment by age, sex, diabetes, revascularization, previous HF, hemoglobin, atrial fibrillation and medical treatment, a continuous risk was observed according to the FIB-4 index (figure 3B). FIB-4 index ≥ 1.30 was independently associated with higher MB risk (sHR, 1.62, 95%CI, 0.105-2.88; P=.030). For patients not at high bleeding risk, the reclassification index of the FIB-4 was 13.27%, net reclassification improvement 0.058 (0.091-1.105) and integrated discrimination improvement 0.02 (0.001-0.060).
FIB-4 index assessment during the acute coronary syndrome hospitalization vs before or afterwardAST, ALT, and platelet count levels previous to the ACS admission was available in 63% of the patients. Median time to the previous blood test was 315.5 [193-522] days before admission. In-hospital results of AST, compared with previously available levels, were 35.7% higher (42 vs 27 U/L; P=.001) and ALT levels were 28.1% higher (23.2 U/L vs 32.2 U/L; P=.001); no differences in platelet count were observed (figure 1 of the supplementary data). All analyses were repeated using AST and ALT levels from blood tests obtained before ACS admission and no significant associations were obtained with any of the outcomes. Post-discharge transaminase levels were available in 75% of the cohort and a decrease of only 8% was observed in AST and ALT; no change was observed in platelet count.
DISCUSSIONThis analysis of a cohort of consecutive patients admitted for ACS demonstrates the impact of liver fibrosis on in-hospital mortality and postdischarge HF and MB (figure 3). The results highlight the predictive value of liver fibrosis for 2 of the most important complications in patients discharged after an ACS. Since clinical features and outcomes are similar to previous reports,21–24 we believe that these results could be representative and translated to clinical practice.
We believe that our results might have clinical implications for therapeutics, such as selection of patients who could be candidates for abbreviated treatment with dual antiplatelet treatment or closer follow-up, as well as for the screening of patients in whom a liver ultrasound should be recommended for further diagnosis
The diagnostic performance of the FIB-4 index during an ACS hospitalization might not be validated. Transaminases could increase in patients with cardiogenic shock or with a high inflammatory response2 and this could have overestimated the prevalence of liver fibrosis. Transaminase levels during ACS admission were significantly higher than previously available levels but postdischarge levels were also higher, although slightly lower than values at admission. The diagnostic performance of FIB-4 has been demonstrated to be highly accurate in different forms of metabolic dysfunction-associated fatty liver disease.4,25 We believe that these results are only hypothesis generating and might reflect the impact of ACS on liver function.
NAFLD, which is preceded by liver fibrosis, will be the leading cause of liver failure and transplant by 2025.26 The assessment of the FIB-4 index is very accessible, is one of the best most precise scores25 and has a good correlation with the gold-standard imaging techniques.22 Nonetheless, its impact on the prognosis of patients with cardiovascular disease, and especially ACS, has not previously been demonstrated. The prospective 4-year follow-up of 1773 adults from the NASH Clinical Research Network, revealed that FIB-4 index> 2.67 conferred a higher risk of all-cause mortality, even despite having no liver decompensations.9 In contrast, NAFLD was not associated with a higher risk of myocardial infarction or stroke in a large cohort of participants without cardiovascular disease.27 Our results show a linear and independent association between the FIB-4 index and in-hospital mortality risk and patients with values> 2.67 with had 2-fold higher mortality rates. Although previous reports suggested that screening for NAFLD in individuals with diabetes might not be cost-effective,28 our data provide compelling evidence of the impact of NAFLD on ACS mortality. Nonetheless, lifestyle modifications and weight loss are highly encouraged in all clinical guidelines for primary and secondary prevention of cardiovascular disease.29
In contrast to in-hospital mortality risk, no effect of the FIB-4 index was observed on long-term mortality. Participants with NAFLD had an increased risk of incident HF, with a higher risk of developing HF with preserved ejection fraction.7 The results of this study also suggested an epidemiological link between NAFLD and HF beyond the basis of shared risk factors due to the persistence of an increased risk after adjustment for clinical and demographic factors, as also highlighted by an American Heart Association statement.4 These results have also been verified in a meta-analysis that demonstrated the higher risk of incident HF in patients with NAFLD8. Our results are fully in agreement with these findings and provide evidence of the independent risk of the FIB-4 index for HF readmissions. Our group previously reported that the risk of HF is much higher than the risk of mortality in ACS patients without previous HF or left ventricle dysfunction30 and this new study supports the relevant role of FIB-4 for the identification of patients at high risk of HF incidence.
The current binary definition of high bleeding risk patients of the Academic Research Consortium for High Bleeding Risk6 does not include mild or moderate liver abnormalities and only cirrhosis with portal hypertension is included as major criteria. Most coagulation factors are synthesized in the liver and subclinical liver dysfunction might be associated with an impairment of these factors.4 This definition has been demonstrated to accurately identify patients at higher risk of MB31 and our group also demonstrated that ACS patients categorized as at high bleeding risk have a higher risk of MB than of all-cause mortality in the first 6 to 7 years after the ACS.32 In this new study, the prevalence of high bleeding risk patients among those with FIB-4> 1.30 was> 50% and, moreover, a FIB-4 index> 1.30 was independently associated with a higher risk of MB after hospital discharge. These results highlight that a relevant percentage of patients classified as non-high bleeding risk by current recommendations had a FIB-4 index> 1.30 and that this is associated with higher risk of MB. Moreover, the net reclassification improvement, representing the average weighted improvement and integrated discrimination improvement, which reflects the improvement in average sensitivity without sacrificing average specificity, were positive. Thereafter, our results support the assessment of the FIB-4 index especially in non-high bleeding risk patients.
These results underscore the impact of mild or moderate liver fibrosis on MB. We believe that such results might reinforce the message of the clinical and prognostic importance of assessing liver fibrosis by the FIB-4 index in all ACS patients.
LimitationsOur study has some limitations that should be addressed. First, as all observational studies, there are some inherent limitations such as the lack of randomization, the long-term variations in medical treatments, or uncontrolled variables. Second, alcohol consumption is not systematically registered in our database and, thereafter, we could not exclude the possible effect of alcohol abuse on liver function. Third, diagnosis of HF onset could also be debated but, since we analyzed only hospital readmissions, we believe that we included real HF cases and did not include cases of dyspnea or breathing disorders. Last, since the inclusion period was long, the use of concurrent treatments might have changed through the inclusion period. Nonetheless, as the clinical features and incidence of long-term events is similar to those of previous reports,21–24,31 we believe that the above-mentioned limitations might not had a relevant impact on our results.
CONCLUSIONSThe assessment of liver fibrosis, by the FIB-4 index, identifies ACS patients not only at higher risk of in-hospital mortality but also at higher risk of HF and MB after discharge. The FIB-4 index is very accessible and, although it is not an established method for the definitive diagnosis of liver fibrosis, it could be used to select patients who should undergo further diagnostic imaging techniques.
- –
Liver fibrosis precedes cirrhosis and severe liver dysfunction.
- –
Liver fibrosis in underlying nonalcoholic fatty liver disease is associated with diabetes, obesity, and cardiovascular disease.
- –
Advanced liver dysfunction is a major risk factor for major bleeding.
- –
Liver fibrosis can be assessed by not only by imaging techniques but also by clinical indexes, such as the FIB-4.
- –
A total of 6.5% of the patients admitted for an ACS have a FIB-4 index> 1.3.
- –
FIB-4 index ≥ 1.3 was associated with a higher risk of first and recurrent heart failure hospitalizations.
- –
FIB-4 index ≥ 1.3 was associated with a higher risk of major bleeding.
This study received the support of the National Network for Biomedical Cardiovascular Research of Cardiovascular Disease (CIBERCV, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares).
AUTHORS’ CONTRIBUTIONSConceptualization and methodology: A. Cordero; formal analysis and original draft: A. Cordero, D. Escribano; investigation: M. A. Quintanilla, J. M. López-Ayala; data collection: M. D. Masía, D. Cazorla, E. Martínez Rey-Rañal, J. Moreno-Arribas; draft review, editing, and supervision: P. Zuazola.
CONFLICTS OF INTERESTA. Cordero reports conferences with AstraZeneca, AMGEN, Bristol-Myers Squibb, Ferrer, Boehringer Ingelheim, MSD, Daichy Sankio, Novartis, Novo Nordisk and Amarin; and consultancy with AstraZeneca, Ferrer, AMGEN, Novartis, Lilly, Novo Nordisk, and Amarin. The rest of the authors have no conflicts of interest.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.12.013