Cardiac troponin, a marker of myocardial injury, is frequently observed in patients with COVID-19 infection. Our objective was to analyze myocardial injury and its prognostic implications in patients with and without COVID-19 infection treated in the same period of time.
MethodsThe present study included patients treated in a university hospital with cardiac troponin I measurements and with suspected COVID-19 infection, confirmed or ruled out by polymerase chain reaction analysis. The impact was analyzed of cardiac troponin I positivity on 30-day mortality.
ResultsIn total, 433 patients were distributed among the following groups: confirmed COVID-19 (n=186), 22% with myocardial injury (n=41); and ruled out COVID-19 (n=247), 21.5% with myocardial injury (n=52). The confirmed and ruled out COVID-19 groups had a similar age, sex, and cardiovascular history. Mortality was significantly higher in the confirmed COVID-19 group than in the ruled out group (19.9% vs 5.3%, P <.001). In Cox multivariate regression analysis, cardiac troponin I was a predictor of mortality in both groups (confirmed COVID-19 group: HR, 3.54; 95%CI, 1.70-7.34; P=.001; ruled out COVID-19 group: HR, 5.57; 95%CI, 1.70-18.20; P=.004). The predictive model analyzed by ROC curves was similar in the 2 groups (P=.701), with AUCs of 0.808 in the confirmed COVID-19 group (0.750-0.865) and 0.812 in the ruled out COVID-19 group (0.760-0.864).
ConclusionsMyocardial injury is detected in 1 in every 5 patients with confirmed or ruled out COVID-19 and predicts 30-day mortality to a similar extent in both circumstances.
Keywords
Coronavirus disease 2019 (COVID-19) is caused by the new coronavirus SARS-CoV-2 and has produced a global pandemic with colossal impact. The disease has a wide spectrum of clinical manifestations, ranging from asymptomatic or minimally symptomatic episodes to severe respiratory failure requiring mechanical ventilation and death.1 A number of prognostic markers have been identified that could facilitate early risk stratification in COVID-19 patients. These markers include advanced age and comorbidities such as cardiovascular disease, chronic lung disease, hypertension, and cancer,2,3 as well as several biochemical markers, including ferritin, leukopenia, and D dimer.4
COVID-19 has major cardiovascular repercussions and causes myocardial injury that can manifest as an increase in cardiac troponins (cTn).5 Several studies have indicated that elevated cTn could be another marker of poor prognosis, and therefore useful for identifying patients at high risk. The implication of cTn is unsurprising because myocardial injury in the absence of type 1 myocardial infarction is a general risk marker present in many clinical processes associated with possible cardiac involvement.6 To date, published studies of myocardial injury and COVID-19 have been conducted in regions where health care systems have been under immense strain due to the pandemic, with hospitals dedicated almost exclusively to the care of COVID-19 patients.7–11 The association between myocardial injury and COVID-19 prognosis has not been tested in regions where the health care burden has been lower and the care of COVID-19 patients has coexisted alongside that of patients with other diagnoses, often with an infectious etiology
METHODSStudy populationA retrospective observational study was conducted of all consecutive patients treated for suspected SARS-CoV-2 infection at a university hospital between March 16 and April 16 2020 and who had at least 1 available cardiac troponin I (cTnI) measurement. cTnI assay was included in the emergency department analytical protocol for patients with suspected COVID-19. Almost all patients were initially treated in the emergency department, and only those with extremely severe symptoms were transferred to the intensive care unit (ICU). Patients with mild clinical symptoms and normal chest X-ray were discharged immediately, without a blood analysis.
Patients were identified for inclusion in the study by examining SARS-CoV-2 polymerase chain reaction (PCR) results and cTnI values for the same patients, determined in the hospital laboratory. When a patient had several cTnI determinations, the highest value was selected. Strong clinical suspicion of COVID-19 was confirmed or ruled out by further tests for SARS-CoV-2 antigens or antibodies.
Analyzed variablesPatient variables included demographic data, cardiovascular medical history and associated risk factors, the reason for attending the emergency department, clinical and analytical variables, electrocardiograms, imaging data (chest X-ray), and data from any other examinations performed. For hospitalized patients, additional variables were the need for ICU admission, the length of stay in the unit, and the need for mechanical ventilation. Clinical data for patients with elevated cTnI were assessed for imbalance between myocardial oxygen supply and demand (increased demand, especially tachycardia, or reduced supply, especially severe hypoxemia or hypotension); patients showing an oxygen imblance were categorized as having type 2 myocardial infarction according to established criteria.12 The study population was distributed into 4 groups according to the confirmation or exclusion of COVID-19 diagnosis and the positive or negative outcome of the cTnI assay.
The main outcome variable was 30-day mortality. Patients were followed up by accessing electronic medical records.
Laboratory assaysSARS-CoV-2 PCR assayViral RNA was purified with the RNeasy Mini Kit in a Qiacube Connect analyzer (QIAGEN, Germany). Reverse transcription PCR (RT-PCR) was performed in a CFX96 Touch System thermocycler (Bio-Rad Laboratories Inc, Hercules, United States) using a commercial kit that amplifies SARS-CoV-2 genes E, N, and RdRP (Allplex 2019-nCoV Assay, Seegene Inc, South Korea).
Antigen assaySARS-CoV-2 antigens were detected by immunochromatography assay (Fluorescence Ag Rapid Test, BIOEASY Biotechnology Co, Ltd, China).
Antibody assayAntibodies to SARS-CoV-2 were detected by immunochemiluminescence assay (COVID-19 VIRCLIA Monotest, Vircell SL, Spain).
Cardiac troponin IcTnI was determined with a high-sensitivity troponin I immunoassay kit (Siemens, Advia Centaur, United States). The lower and upper detection limits were 2.5 and 25 000 ng/L, respectively, as established by the manufacturer. Determinations below the lower detection limit were assigned a value of 0 ng/L, and determinations above the upper limit were assigned a value of 25 000 ng/L. The reference limit for a positive cTnI determination was> 47 ng/L, which corresponds to the 99th percentile with a total analytical imprecision <10% as expressed by the coefficient of variation.
Statistical analysisCategorical variables are presented as number (%) and continuous variables as median [interquartile range]. Comparisons between categorical variables were made by chi-square test or the Fisher exact test, as appropriate. Continuous variables were compared by the Mann-Whitney U test. Survival was analyzed with the Kaplan-Meier method, and between-group comparisons were made using the log-rank test. To determine the association between myocardial injury and mortality, the groups with and without COVID-19 were analyzed by univariate and multivariate Cox regression. The adjusted model included variables showing significance in the univariate analysis or other clinically relevant variables. To prevent overadjustment, the model was restricted to 6 variables: age, history of hypertension, history of myocardial infarction, history of chronic lung disease, glomerular filtration rate on admission, and the presence of elevated cTnI. Univariate and multivariate Cox regression analyses were also conducted to assess the interaction between COVID-19 and elevated cTnI in the entire cohort. The proportionality assumption was verified using Schoenfeld residuals. The ability of cTnI to improve mortality prediction in the confirmed or ruled out COVID-19 groups was assessed using a specially designed simple clinical model comprising age, history of hypertension, and glomerular filtration rate on admission. The predictive ability of this model was tested before and after adding cTnI by analysis of the ROC curve, the net reclassification improvement index, and the integrated discrimination improvement index. Finally, the ROC curve method was used to compare the predictive model between the 2 groups. All statistical analyses were performed using the STATA 14.2 statistical program (StatCorp, College Station, United States). Differences were considered statistically significant at P <.05.
This study forms part of a large research project investigating myocardial injury detected in patients treated in the emergency department and has local Ethics Committee approval.
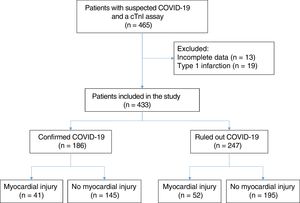
RESULTSDuring the study period, 2447 SARS-CoV-2 PCR tests were performed on samples from 1795 patients. A large number of these tests were on outpatients or patients from other catchment populations. In total, 26% of the samples were from patients treated at our hospital and who had at least one cTnI measurement. Patients with incomplete clinical or analytical data were excluded, as were those with a diagnosis of type 1 myocardial infarction, no suspicion of COVID-19, or a negative PCR assay. The final sample included 433 patients, of whom 186 (43%) had a confirmed COVID-19 diagnosis; 29 of these patients had a negative SARS-CoV-2 PCR assay but a positive serological antigen or antibody assay. COVID-19 infection was ruled out in the remaining 247 patients (57%). Elevated cTnI was detected in 41 patients (22%) in the confirmed COVID-19 group and in 52 patients (21%) in the ruled out COVID-19 group (figure 1).
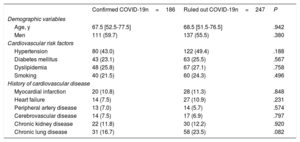
Demographic data, cardiovascular risk factors, and cardiovascular disease histories are shown for the confirmed and ruled out COVID-19 patient groups in table 1. Differences between patients with and without myocardial injury are shown in the table 1of the supplementary data and the table 2 of the supplementary data. Baseline patient characteristics categorized by 30-day survival for the whole cohort (confirmed and ruled out COVID-19 infection) are shown in the table 3 of the supplementary data. The variables showing an association with myocardial injury were similar in both patient groups: age, hypertension, dyslipidemia, a history of myocardial infarction, and a history of kidney disease. The main diagnoses among patients who tested negative for COVID-19 were respiratory infection (48%), other infections (12.2%), heart failure (8.1%), gastrointestinal disease (3.4%), neurological disorders (3.6%), and various other diagnoses.
Baseline characteristics of patients in the confirmed and ruled out COVID-19 groups
| Confirmed COVID-19n=186 | Ruled out COVID-19n=247 | P | |
|---|---|---|---|
| Demographic variables | |||
| Age, y | 67.5 [52.5-77.5] | 68.5 [51.5-76.5] | .942 |
| Men | 111 (59.7) | 137 (55.5) | .380 |
| Cardiovascular risk factors | |||
| Hypertension | 80 (43.0) | 122 (49.4) | .188 |
| Diabetes mellitus | 43 (23.1) | 63 (25.5) | .567 |
| Dyslipidemia | 48 (25.8) | 67 (27.1) | .758 |
| Smoking | 40 (21.5) | 60 (24.3) | .496 |
| History of cardiovascular disease | |||
| Myocardial infarction | 20 (10.8) | 28 (11.3) | .848 |
| Heart failure | 14 (7.5) | 27 (10.9) | .231 |
| Peripheral artery disease | 13 (7.0) | 14 (5.7) | .574 |
| Cerebrovascular disease | 14 (7.5) | 17 (6.9) | .797 |
| Chronic kidney disease | 22 (11.8) | 30 (12.2) | .920 |
| Chronic lung disease | 31 (16.7) | 58 (23.5) | .082 |
Data are expressed as No. (%) or median [interquartile range].
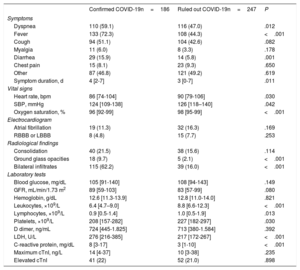
Patients with confirmed COVID-19 more frequently presented with dyspnea, fever, and diarrhea, but the rates of coughing and muscle pain were similar to those in patients without COVID-19 (table 2 and the tables 4–6 of the supplementary data). The initial vital signs of confirmed COVID-19 patients showed small but significant differences from those of patients without COVID-19 (lower heart rate, lower systolic blood pressure, and lower oxygen saturation). Chest X-rays revealed a pattern of bilateral infiltration in almost 2 out of every 3 confirmed COVID-19 patients. Patients with and without COVID-19 showed no differences in baseline blood glucose, glomerular filtration rate, or hemoglobin. However, the 2 patient groups differed in the remaining analytical parameters. Confirmed COVID-19 patients had lower leukocyte, lymphocyte, and platelet counts, and while D dimer values were similar in the 2 groups, confirmed COVID-19 patients had higher levels of serum lactate dehydrogenase and C-reactive protein.
Clinical characteristics of patients in the confirmed and ruled out COVID-19 groups
| Confirmed COVID-19n=186 | Ruled out COVID-19n=247 | P | |
|---|---|---|---|
| Symptoms | |||
| Dyspnea | 110 (59.1) | 116 (47.0) | .012 |
| Fever | 133 (72.3) | 108 (44.3) | <.001 |
| Cough | 94 (51.1) | 104 (42.6) | .082 |
| Myalgia | 11 (6.0) | 8 (3.3) | .178 |
| Diarrhea | 29 (15.9) | 14 (5.8) | .001 |
| Chest pain | 15 (8.1) | 23 (9.3) | .650 |
| Other | 87 (46.8) | 121 (49.2) | .619 |
| Symptom duration, d | 4 [2-7] | 3 [0-7] | .011 |
| Vital signs | |||
| Heart rate, bpm | 86 [74-104] | 90 [79-106] | .030 |
| SBP, mmHg | 124 [109-138] | 126 [118–140] | .042 |
| Oxygen saturation, % | 96 [92-99] | 98 [95-99] | <.001 |
| Electrocardiogram | |||
| Atrial fibrillation | 19 (11.3) | 32 (16.3) | .169 |
| RBBB or LBBB | 8 (4.8) | 15 (7.7) | .253 |
| Radiological findings | |||
| Consolidation | 40 (21.5) | 38 (15.6) | .114 |
| Ground glass opacities | 18 (9.7) | 5 (2.1) | <.001 |
| Bilateral infiltrates | 115 (62.2) | 39 (16.0) | <.001 |
| Laboratory tests | |||
| Blood glucose, mg/dL | 105 [91-140] | 108 [94-143] | .149 |
| GFR, mL/min/1.73 m2 | 89 [59-103] | 83 [57-99] | .080 |
| Hemoglobin, g/dL | 12.6 [11.3-13.9] | 12.8 [11.0-14.0] | .821 |
| Leukocytes, ×109/L | 6.4 [4.7–9.0] | 8.8 [6.6-12.3] | <.001 |
| Lymphocytes, ×109/L | 0.9 [0.5-1.4] | 1.0 [0.5-1.9] | .013 |
| Platelets, ×109/L | 208 [157-282] | 227 [182-297] | .030 |
| D dimer, ng/mL | 724 [445-1.825] | 713 [380-1.584] | .392 |
| LDH, U/L | 276 [216-385] | 217 [172-267] | <.001 |
| C-reactive protein, mg/dL | 8 [3-17] | 3 [1-10] | <.001 |
| Maximum cTnI, ng/L | 14 [4-37] | 10 [3-38] | .235 |
| Elevated cTnI | 41 (22) | 52 (21.0) | .898 |
cTnI, cardiac troponin I; GFR, glomerular filtration rate; LBBB, left bundle branch block; LDH, lactate dehydrogenase; RBBB, right bundle branch block; SBP, systolic blood pressure.
Data are expressed as No. (%) or median [interquartile range].
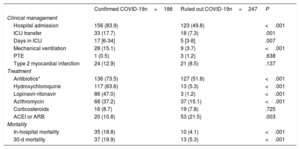
Patients with a confirmed COVID-19 diagnosis had a higher rate of hospitalization and ICU admission (mean admission time, 17 days). Confirmed COVID-19 patients also more frequently required mechanical ventilation (table 3 and table 7 of the supplementary data and table 8 of the supplementary data). Patients with and without COVID-19 showed no differences in the rate of pulmonary thromboembolism (0.5% and 1.2%, respectively) or the diagnosis of type 2 myocardial infarction (12.9% and 8.1%).
Clinical management, treatment, and mortality among patients in the confirmed and ruled out COVID-19 groups
| Confirmed COVID-19n=186 | Ruled out COVID-19n=247 | P | |
|---|---|---|---|
| Clinical management | |||
| Hospital admission | 156 (83.9) | 123 (49.8) | <.001 |
| ICU transfer | 33 (17.7) | 18 (7.3) | .001 |
| Days in ICU | 17 [6-34] | 5 [3-8] | .007 |
| Mechanical ventilation | 28 (15.1) | 9 (3.7) | <.001 |
| PTE | 1 (0.5) | 3 (1.2) | .638 |
| Type 2 myocardial infarction | 24 (12.9) | 21 (8.5) | .137 |
| Treatment | |||
| Antibiotics* | 136 (73.5) | 127 (51.8) | <.001 |
| Hydroxychloroquine | 117 (63.6) | 13 (5.3) | <.001 |
| Lopinavir-ritonavir | 86 (47.0) | 3 (1.2) | <.001 |
| Azithromycin | 68 (37.2) | 37 (15.1) | <.001 |
| Corticosteroids | 16 (8.7) | 19 (7.8) | .725 |
| ACEI or ARB | 20 (10.8) | 53 (21.5) | .003 |
| Mortality | |||
| In-hospital mortality | 35 (18.8) | 10 (4.1) | <.001 |
| 30-d mortality | 37 (19.9) | 13 (5.3) | <.001 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ICU, intensive care unit; PTE, pulmonary thromboembolism.
Data are expressed as No. (%) or median [interquartile range].
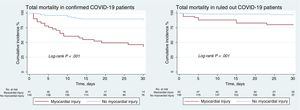
Treatments differed significantly between the 2 groups. Antibiotics were administered to 73% of confirmed COVID-19 patients, and two thirds of these patients were treated with hydroxychloroquine, almost half with lopinavir-ritonavir, one third with azithromycin, and a handful with corticosteroids (table 3). In-hospital mortality in the confirmed COVID-19 group was 18.8%, and 53.7% of these deceased patients had myocardial injury. In-hospital mortality among patients without COVID-19 was 4.1%, with myocardial injury detected in 13.5% of these patients (figure 2). The variables showing a statistical association with 30-day mortality among confirmed COVID-19 patients in the multivariate Cox logistic regression analysis were a history of chronic lung disease, glomerular filtration rate on admission, and a positive cTnI assay; for the ruled out COVID-19 group, the statistically associated variables were age, a history of myocardial infarction, and a positive cTnI assay (table 4). As these observations indicate, COVID-19 infection and a positive cTnI assay showed no interaction in 30-day mortality.
Univariate and multivariate analyses of 30-day mortality in the whole cohort and in the confirmed and ruled out COVID-19 patient groups
| Confirmed COVID-19 and ruled out COVID-19 | ||||
|---|---|---|---|---|
| Univariate Cox regression | Multivariate Cox regression | |||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.05 (1.03-1.08) | <.001 | 1.03 (1.01-1.05) | .041 |
| Hypertension | 3.13 (1.69-5.80) | <.001 | - | - |
| History of AMI | 3.15 (1.67-5.93) | <.001 | - | - |
| History of CLD | 1.87 (1.03-3.38) | .040 | 1.85 (1.01-3.41) | .047 |
| GFR on admission* | 1.03 (1.02-1.04) | <.001 | 1.02 (1.01-1.03) | <.001 |
| COVID-19 | 3.53 (1.87-6.64) | <.001 | 3.59 (1.62-7.93) | .002 |
| Myocardial injury | 6.50 (3.67-11.51) | <.001 | 4.27 (1.28-14.22) | .018 |
| Interaction between myocardial injury and COVID-19 | 4.43 (1.47-13.34) | .008 | 1.45 (0.37-5.65) | .590 |
| Confirmed COVID-19 | ||||
|---|---|---|---|---|
| Univariate Cox regression | Multivariate Cox regression | |||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.05 (1.02-1.07) | <.001 | - | - |
| Hypertension | 2.93 (1.47-5.83) | .002 | - | - |
| History of AMI | 2.69 (1.23-5.89) | .013 | - | - |
| History of CLD | 3.03 (1.54-5.95) | .001 | 2.57 (1.30-5.09) | .007 |
| GFR on admission* | 1.03 (1.02-1.04) | <.001 | 1.02 (1.01-1.03) | <.001 |
| Myocardial injury | 6.80 (3.52-13.13) | <.001 | 3.54 (1.70-7.34) | .001 |
| Ruled out COVID-19 | ||||
|---|---|---|---|---|
| Univariate Cox regression | Multivariate Cox regression | |||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.07 (1.02-1.12) | .003 | 1.06 (1.01-1.12) | .021 |
| Hypertension | 6.00 (1.33-27.09) | .020 | - | - |
| History of AMI | 5.08 (1.66-15.53) | .004 | 3.09 (1.01-9.51) | .049 |
| History of CLD | 0.94 (0.26-3.42) | .930 | - | - |
| GFR on admission* | 1.02 (1.01-1.04) | .003 | - | - |
| Myocardial injury | 8.19 (2.52-26.62) | <.001 | 5.57 (1.70-18.20) | .004 |
95%CI, 95% confidence interval; AMI, acute myocardial infarction; CLD, chronic lung disease; GFR, glomerular filtration rate; HR, hazard ratio.
For the confirmed COVID-19 group, ROC curve analysis of 30-day mortality in the clinical predictive model revealed an area under the curve (AUC)=0.775 (95% confidence interval [95%CI], 0.716-0.835). The addition of cTnI increased the AUC to 0.808 (95%CI, 0.750-0.865), and the difference was statistically significant (P=.024). Adding cTnI to the clinical model also increased net reclassification improvement by 0.632 (0.285-0.979; P <.001) and integrated discrimination improvement by 0.039 (0.005-0.073; P=.013).
For the ruled out COVID-19 group, ROC curve analysis of 30-day mortality in the clinical predictive model yielded an AUC=0.770 (95%CI, 0.713-0.826), and adding cTnI increased the AUC to 0.812 (95%CI, 0.760-0.864; P=.023). Adding cTnI to the clinical model increased net reclassification improvement by 1.058 (0.519-1.597; P <.001) and integrated discrimination improvement by 0.068 (0.032-0.103; P<.001).
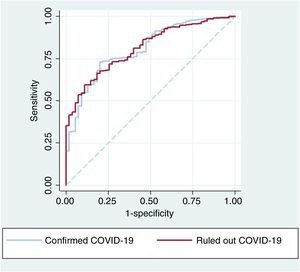
The ROC curves for the predictive model including cTnI did not differ significantly between the confirmed and ruled out COVID-19 groups (P=.701) (figure 3).
DISCUSSIONMain findingsOur study shows that in a consecutive cohort with suspected COVID-19, patients with positive and negative SARS-CoV-2 PCR results had a similar degree of myocardial injury assessed by cTnI assay. The predictive ability of elevated cTnI to identify 30-day mortality risk was similar in the 2 patient groups. The higher mortality in the confirmed COVID-19 group must therefore be due to mechanisms independent of cardiac involvement.
Myocardial injury in patients with confirmed COVID-19Mortality is high among COVID-19 patients requiring hospitalization and is even higher among those who are elderly or have a history of underlying cardiovascular disease.13 An association between SARS-CoV-2 infection and myocardial injury was first reported in a small series of 41 hospitalized COVID-19 patients, 5 of whom had elevated cTnI.7 Elevated cTnI in COVID-19 patients was later also reported in several other small studies of Chinese populations.7–11 In a cohort of 191 patients with confirmed COVID-19, univariate analysis indicated that the risk of death was higher when the cTnI concentration exceeded the 99th percentile upper reference limit (odds ratio [OR]=80.15; 95%CI, 10.3-620.4; P <.0001).3 These patients had a higher requirement for invasive and noninvasive ventilation (22% vs 4% and 46% vs 4%, respectively), as well as higher rates of acute respiratory distress syndrome (59% vs 15%) and acute kidney failure (9% vs 0%) (P <.001 in all cases). Mortality was 10 times higher among patients with myocardial injury at presentation (51% vs 5%; hazard ratio [HR]=3.41; 95%CI, 1.62-7.16). With the global spread of COVID-19 from China, an association with myocardial injury has also been described in other countries, including Italy14 and the United States.15 Possible mechanisms of myocardial injury and related cardiac phenotypes in COVID-19 include a direct action of the virus on the myocardium, coronary microvascular ischemia mediated by the binding of SARS-CoV-2 to endothelial angiotensin II converting enzyme II (ACE2), stress cardiomyopathy, and tachycardia due to exogenous adrenergic stimulation.16
Studies published to date may have overestimated the prevalence of myocardial injury among COVID-19 patients. cTnI values were available for 145 of 191 patients (75%) included in a series of 813 consecutive adults admitted to Jinyintan Hospital or Wuhan Pulmonary Hospital3 and for 416 of 645 consecutive patients (64%) admitted to Renmin Hospital of Wuhan University.10 These rates suggest that cTnI was likely measured only in patients with suspected cardiac involvement (myocardial ischemia or ventricular dysfunction). However, although cTnI was measured systematically in all patients in our series, the proportion of patients with elevated cTnI was similar to that reported in the Chinese studies, indicating similar rates of myocardial injury. Moreover, although no instances were detected in our series, elevated cTnI is sometimes associated with type 1 myocardial infarction due to atherothrombosis triggered by the proinflammatory and prothrombotic state, as described in previous influenza epidemics17 and other inflammatory situations.18 Among patients with severe hypoxemia, hypotension, or prolonged tachycardia, type 2 myocardial infarction is more common. In our series, more than half of the confirmed COVID-19 patients met the criteria for type 2 myocardial infarction, a finding not reported in previous studies.
Cardiovascular risk factors and comorbidities are prevalent in COVID-19 patients,19 and while these conditions do not appear to enhance SARS-CoV-2 infectiousness, they may increase disease severity. An important goal of cardiovascular therapy is to reduce the concentration or activity of angiotensin II, which is involved in inflammatory mechanisms and endothelial dysfunction. It will be important to clarify whether treatment-induced tissue overexpression of ACE2 enhances SARS-CoV-2 infection or overcomes the ACE2 deficit to reduce inflammation and vasoconstriction in the heart, lungs, and kidneys. Likewise, studies should address the regulation of serum ACE2 concentration and its ability to reduce the affinity of SARS-CoV-2 for tissue ACE2 and thereby reduce infection. A study of 18 422 patients tested for COVID-19 (24.5% positive; 9.3% requiring hospitalization) observed no association between treatment with ACE inhibitors or angiotensin II receptor antagonists and a positive SARS-CoV-2 test result.20
It is unclear if the presumably acute myocardial injury detected in COVID-19 patients will cause future chronic myocardial injury and structural coronary disease. However, a study of 25 survivors of the previous SARS coronavirus epidemic 12 years after their clinical recovery found that 64% had hyperlipidemia, 44% cardiovascular abnormalities, and 60% glucose metabolism alterations; a metabolomic analysis of these patients revealed dysregulation of lipid metabolism.21
The detection of myocardial injury could facilitate appropriate decision making about transfer to the ICU, improve understanding of the systemic consequences of COVID-19, and guide medication with drugs such as inotropes, vasopressors, and diuretics for patients with significant cardiac dysfunction. Furthermore, additional examination by echocardiography or cardiac magnetic resonance could help to identify and guide therapy for COVID-19 survivors with a clearly defined cardiac phenotype.
Myocardial injury in patients without COVID-19The most remarkable findings of our study are likely the high frequency of myocardial injury in conditions not caused by COVID-19 and the similar predictive ability of cTnI in patients with and without COVID-19. This is not surprising, since a previous study found a similar incidence of myocardial injury in patients without type 1 myocardial infarction to that reported here.6 It is important to define the pathophysiological diagnosis of these patients according to the fourth universal definition of myocardial infarction22 as type 2 myocardial infarction12 or as acute or chronic nonischemic myocardial injury.23 Whatever the cause of hospitalization, admitted patients often have an imbalance in oxygen supply and demand, and this is especially common among patients in a critical condition. This oxygen deficit is not limited to the myocardium and likely occurs in the cells of most organs. However, the sensitivity of cTnI assays ensures that cTnI is one of the earliest detected and most accurate biomarkers of organ dysfunction. In this context, cTnI testing could provide the basis for the early initiation of treatments to improve general tissue oxygenation and perfusion.
In recently published preliminary guidelines on the use of biomarkers in COVID-19 patients, the American College of Cardiology recommends cTnI measurement only in patients with suspected myocardial infarction.24 However, understanding the effect of COVID-19 on the cardiovascular system is essential to provide appropriate and comprehensive treatment to patients with and without a history of heart disease. Accurate estimates of the prevalence of myocardial injury in COVID-19 can be obtained only through the systematic testing of asymptomatic and symptomatic individuals infected with SARS-CoV-2.25
LimitationsOur study has some limitations. First, in most patients, cTnI was determined on or within 24hours of admission, with no repeat measurements over the course of the disease. However, changes in cTnI values have been reported during the hospitalization of COVID-19 patients.3 A second limitation is that echocardiography was performed only sporadically, and we therefore lack information about the possible repercussions of myocardial injury on ventricular function in these patients. Third, COVID-19 was diagnosed based on PCR and serological test results. Although these tests are highly sensitive and specific, we cannot exclude the possibility of false-positive and false-negative results in our study population. However, this possibility seems unlikely given that all patients were followed up for at least 1 month. Finally, the ruled out COVID-19 group was relatively heterogeneous, although infectious disease predominated, especially affecting the respiratory system.
CONCLUSIONSA number of questions remain regarding the myocardial injury detected in COVID-19. Areas of uncertainty include the mechanism linking SARS-CoV-2 to myocardial injury, how myocardial injury in COVID-19 patients differs from that detected in other populations, and what specific therapeutic options are available for myocardial injury in COVID-19.26 Our study makes an important contribution to answering one of these questions; myocardial injury in COVID-19 patients is unlikely to differ significantly from that present in multiple acute processes, whether infectious or sterile, and the impact on prognosis is also likely similar. Future efforts should therefore be directed at defining the mechanisms of myocardial injury in patients with acute conditions and advancing the development of strategies to mitigate the associated poor prognosis.
FUNDINGThis project was partly supported by a FIS grant within the program Proyectos de Investigación en Salud, Acción Estratégica en Salud 2017-2020, PI19/00705.
CONFLICTS OF INTERESTThe authors declare that they have no conflicts of interest.
- -
COVID-19 mainly affects the respiratory system, manifesting as pneumonia. However, this disease has a broad spectrum of clinical manifestations, ranging from asymptomatic or mildly symptomatic to extremely severe episodes. Myocardial injury detected as an increase in cTn is one of the main factors associated with mortality in this disease.
- -
The frequency of myocardial injury assessed by elevated cTnI was similar in patients with confirmed COVID-19 to that in patients with exclusion of suspected COVID-19 and who were treated at the same hospital and in the same period. In both patient groups, myocardial injury was an important predictor of in-hospital mortality. A model including clinical variables and cardiac troponin showed a similar ability to predict in-hospital mortality in both patient groups.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.08.027