In moderate or high risk non-ST segment elevation acute coronary syndrome, clinical practice guidelines recommend a coronary angiography with intent to revascularize. However, evidence to support this recommendation in very elderly patients is poor.
MethodsAll patients over 85 years old admitted to our hospital between 2004 and 2009 with a diagnosis of non-ST segment elevation acute coronary syndrome were retrospectively included. Using a propensity score, patients undergoing the interventional approach and those undergoing conservative management were matched and compared for survival and survival without ischemic events.
ResultsWe included 228 consecutive patients with a mean age of 88 years (range: 85 to 101). Those in the interventional approach group (n=100) were younger, with a higher proportion of males and less comorbidity, less cognitive impairment and lower troponin I levels compared with patients in the conservative management group (n=128). We matched 63 patients from the interventional approach group and 63 from the conservative management group using propensity score.
In the matched patients, the interventional approach group exhibited better survival (log rank 4.24; P=.039) and better survival free of ischemic events (log rank 8.63; P=.003) at the 3-year follow-up. In the whole population, adjusted for propensity score quintiles, the interventional approach group had lower mortality (hazard ratio 0.52; 95% confidence interval: 0.32-0.85) and a better survival free of ischemic events (hazard ratio 0.48; 95% confidence interval: 0.32-0.74).
ConclusionsNearly all the very elderly patients admitted with non-ST segment elevation acute coronary syndrome were of moderate or high risk. In these patients, the interventional approach was associated with overall better survival and better survival free of ischemic events.
Keywords
The incidence of ischemic heart disease increases with age and, given the progressive ageing of the population in the last decade,1 elderly patients represent a growing percentage of hospitalizations for acute coronary syndrome. According to the GRACE (Global Registry of Acute Coronary Events) registry, it is estimated that about 20% of patients suffering an acute coronary event are more than 75 years old and 6% are more than 85 years old.2 This prevalence contrasts with the low presence of these patients in clinical trials, with only 9% of patients in clinical trials between 1991 and 2000 being more than 75 years of age.3
Patients included in clinical trials are carefully selected according to stringent inclusion criteria,4 with patients at lower risk and with less comorbidity than in real life being recorded.5 Therefore, the applicability of the clinical trial results including those on record may be limited in clinical practice for very elderly patients with high comorbidity.
The European Society of Cardiology6 guidelines emphasize risk stratification for therapeutic decision making. Patients with moderate to high risk are recommended a routine coronary angiography, while those at low risk only if there is recurrent ischemia or an ischemic test is positive. Intervening less in patients at high risk has been associated with a worse prognosis at our center.7 The subgroup of very elderly patients has the highest risk and mortality rates,8 and can therefore benefit most from such therapy. However, these patients are also at increased risk of complications of invasive procedures, which may lead to less aggressive management in daily clinical practice, meaning that they do not benefit as much from the treatments recommended by the guidelines, including coronary angiography.9, 10
There are few comparative data and no randomized trials on the treatment of non-ST segment elevation acute coronary syndrome (NSTE-ACS) in the very elderly. The aim of our study was to assess whether the interventional approach (IA), defined as coronary angiography during hospitalization with intent to revascularize, was better than conservative management (CM) in these patients, by analyzing complications during hospitalization and the medium-term prognosis.
METHODSA retrospective analysis was performed of patients aged ≥85 years admitted consecutively between 1 January 2004 and 31 December 2009 with a diagnosis of NSTE-ACS. The study was conducted in a hospital with a cardiac catheterization laboratory.
All hospitalized patients with a NSTE-ACS diagnosis were included: unstable, ischemic chest pain (at rest; or of recent occurrence [last month]) during effort; or with decreased threshold in the last month), with or without elevated markers of myocardial damage and electrocardiographic (ECG) changes different from persistent ST-segment elevation or left bundle branch block of recent onset.
A review of hospital medical records and electronic databases was performed, generating a database with more than 200 variables concerning demographics, medical history, clinical features, physical examinations, laboratory test results, ECG features and therapeutic management. For the patients who underwent intervention, the angiographic variables and features of the procedure were recorded.
The Charlson comorbidity index without age11 at the time of admission was calculated, using a cut-off of >3 points to define a high level of comorbidity.
The risk for all patients was stratified using the GRACE risk score at admission, as recommended by the European Society of Cardiology guidelines.6, 12 High risk was defined as more than 140 points and intermediate risk more than 109 points.6, 12
Complications occurring during hospitalization were recorded, which included the development of renal failure, TIMI bleeding, the need for transfusion and the occurrence of new myocardial infarction, stroke or death.
DefinitionsA successful procedure was defined as the treated artery being open, with residual stenosis <50% and a final TIMI III flow in the absence of complications (coronary perforation or dissection).
Renal function was calculated by estimating the glomerular filtration rate using the MDRD (Modification of Diet in Renal Disease) formula.13 Renal function deterioration at discharge was defined as a worsening in the creatinine value of >0.5g/dL compared with the value at admission.
Recurrent ischemia was defined as recurrence of angina pain during hospitalization, even if there were no electrical changes or new elevation of troponin.
TIMI bleeding was defined as stated above,14 with major bleeding being any intracranial hemorrhage or clinical bleeding with a decrease in hemoglobin of ≥5g/dL, while minor bleeding was clinical bleeding with a decrease in the hemoglobin value of between 3 and 5g/dL. The need for transfusion of packed red blood cells during hospitalization was also recorded.
Myocardial infarction was defined as a new elevation of troponin associated with a new clinical episode. Stroke was the occurrence of a new neurological deficit accompanied by compatible imaging test (computed tomography or magnetic resonance imaging).
All follow-ups were performed in October 2010 by reviewing medical records, hospital databases and telephone interviews. An event during follow-up was defined as the combination of death from any cause or major acute cardiovascular event (MACE), readmission due to a new acute coronary syndrome, the need for revascularization or stroke. Re-admissions for heart failure or hemorrhage were also recorded.
Statistical AnalysisQuantitative variables were expressed as mean±standard deviation, using the Student's t-test to compare the two groups. Qualitative variables were expressed as a percentage and compared using the chi-square test, or the Fisher's exact test if the expected value in a box was < 5.
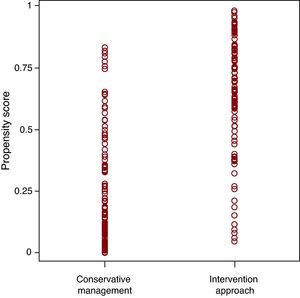
Because the patients in our study were not randomized, we used the propensity score (PS) methodology15 to minimize the expected significant bias between the two groups. We constructed a non-parsimonious logistic regression model in which the dependent variable was cardiac catheterization performed during hospitalization, and the independent variables were those that showed significant differences in the univariate analysis and multiple comorbidities associated with mortality. The covariates introduced in the study were: age, sex, dyslipidemia, previous myocardial infarction, hemoglobin, severe valvular disease, recurrent angina, prior stroke, peripheral arterial disease, dementia, renal failure, Charlson comorbidity index, positive troponin I and the GRACE score. The constructed model discriminated well between both groups with a C statistic of 0.854 (95% confidence interval [CI], 0.804-0.903). Figure 1 shows the PS distribution in each group.
Figure 1. Propensity score distribution by treatment group. Conservative management group: median 0.18; 25 and 75 percentile of 0.076 and 0.476. Interventional aproach group: median 0.68; 25 and 75 percentile of 0.51 and 0.84.
The probability of undergoing IA estimated by the model was used in two ways:
• Firstly, we matched patients in both groups at 1:1 ratio, without replacement. The criterion for matching the pairs was that the difference between the probabilities estimated by the model should be less than 20% of the probability standard deviation, which was 0.30 (20%=0.06) in our study.16
To assess whether the match balanced the baseline characteristics between the two groups, we used the standardized difference17 (Table 1). Small absolute values (≤10%) supported the assumption of balance between groups.18
Table 1. Study Sample Baseline Characteristics and after Matching in the Conservative Management; and Interventional Approach Groups
Total sample Matched sample CMN=128 IAN=100 P CMN=63 IAN=63 Standardised difference (%) Age, years 89±3.4 87±2.4 <.001 87.4±2.5 87.3±2.8 3.8 Age>90 years 50/128 (39.1) 11/100 (11) <.001 10/63 (15.9) 10/63 (15.9) 0 Women 85/128 (66.4) 46/100 (46) .002 34/63 (54) 36/63 (57.1) 6.2 Cardiovascular risk factors Hypertension 95/128 (74.2) 79/100 (79) .69 48/63 (76.2) 49/63 (77.8) 3.8 Diabetes 35/128 (27.3) 25/100 (25) .39 19/63 (30.2) 20/63 (31.7) 3.2 Dyslipidemia 40/128 (31.2) 48/100 (48) .01 24/63 (38.1) 26/63 (41.3) 6.5 Active smoking 2/128 (1.6) 1/100 (1) .71 2/63 (3.2) 1/63 (1.6) 10 >2 CVRF 16/128 (12.5) 14/100 (14) .74 10/63 (15.9) 10/63 (15.9) 0 Cardiovacular history Previous AMI 31/128 (24.2) 38/100 (38) .02 20/63 (31.7) 22/63 (34.9) 6.8 Previous PCI 6/128 (4.7) 13/100 (13) .02 6/63 (9.5) 7/63 (11.1) 5.3 Previous CABG 3/128 (2.3) 5/100 (5) .27 2/63 (3.2) 2/63 (3.2) 0 Previous CHF 28/128 (21.9) 13/100 (13) .08 11/63 (17.5) 11/63 (17.5) 0 Previous AF/Flutter 34/128 (26.6) 16/100 (16) .05 8/63 (12.7) 7/63 (11.1) 4.9 Previous stroke 18/128 (14.1) 16/100 (16) .68 7/63 (11.1) 9/63 (14.3) 9.6 Peripheral arterial disease 12/128 (9.4) 13/100 (13) .38 8/63 (12.7) 9/63 (14.3) 4.7 Comorbidity COPD 19/128 (14.8) 10/100 (10) .27 8/63 (12.7) 8/63 (12.7) 0 Dementia 21/128 (16.4) 4/100 (4) .003 5/63 (7.9) 4/63 (6.3) 6.2 CRF 24/128 (18.8) 24/100 (24) .33 11/63 (17.5) 11/63 (17.5) 0 Cancer 26/128 (20.3) 16/100 (16) .41 12/63 (19) 11/63 (17.5) 3.9 Hemiplegia 6/128 (4.7) 4/100 (4) .81 1/63 (1.6) 2/63 (3.2) 10 Depression (treated) 10/114 (8.8) 8/95 (8.4) .92 4/58 (6.9) 6/61 (9.8) 10 Charlson index 3.3±2.0 2.8±1.9 .10 2.97±1.98 3.06±2.04 4 Charlson index>3 56/128 (43.8) 27/100 (27) .009 23/63 (36.5) 22/63 (34.9) 3.3 Admission characteristics Recurrent ischaemia 28/128 (21.9) 41/100 (41) .002 14/63 (22.2) 16/63 (25.4) 7.5 Troponin I positive 111/128 (86.7) 71/100 (71) .001 52/63 (82.5) 50/63 (79.4) 7.9 ST depression 48/128 (37.5) 40/94 (42.6) .37 26/63 (41.3) 22/60 (36.7) 9.4 Killip class>1 66/128 (51.6) 29/98 (29.6) <.001 21/63 (33.3) 22/62 (35.5) 4.8 Previous ASA 53/127 (41.7) 50/96 (52.1) .127 32/62 (51.6) 30/61 (49.2) 5 Creatinine 1.48±0.67 1.37±0.49 .24 1.34±0.49 1.30±0.43 8.6 Creatinine clearance (MDRD) (mg/dL/1.73m2) 46.7±19.4 51.2±17.7 .12 52.6±19.2 51.6±15.9 5.9 Hb (mg/dL) 12.0±1.8 12.1±1.9 .48 12.11±2.11 12.18±1.69 3.7 LVEF 53±11 52±10.5 .57 54±10.6 53±10.1 9.6 LVEF <35% 9/100 (9) 8/89 (9) .99 4/53 (7.5) 3/58 (5.2) 9.4 VHD (≥3+) 47/100 (47) 29/89 (32.6) .04 16/53 (30.2) 18/58 (31) 1.7 Aortic stenosis (≥3+) 26/100 (26) 15/89 (16.9) .13 9/53 (17) 12/58 (20.7) 9.5 GRACE moderate risk 18/128 (14.1) 16/100 (16) .68 11/63 (17.5) 11/63 (17.5) 0 GRACE high risk 110/128 (85.9) 79/100 (79) .17 52/63 (82.5) 50/63 (79.4) 7.9 AF, atrial fibrillation; AMI, acute myocardial infarction; ASA, acetylsalicylic acid; CABG, coronary artery bypass graft; CHF, congestive heart failure; CM, conservative management; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CVRF, cardiovascular risk factor; GRACE, global registry of acute coronary events; Hb, haemoglobin; IA, interventional approach; LVEF, left ventricle ejection fraction; MDRD, Modification of Diet in Renal Disease; PCI, percutaneous coronary intervention; VHD, valvular heart disease.
Results are expressed as mean±standard deviation or n/N (%).Survival was analyzed with the Kaplan-Meier method, using the log rank test for comparison between groups.
• Secondly, all patients were classified into quintiles according to the probability predicted by the model. The hazard ratio (HR) of the events at follow-up was calculated using a Cox regression model adjusted for PS quintiles. The HR adjusted for year of admission (2004 to 2006 vs 2007 to 2009) was also calculated.
A P value <.05 was considered statistically significant. The statistical analysis was performed using SPSS version 15.0 for Windows (SPSS Inc, Chicago, Illinois, United States).
RESULTSA total of 228 patients aged 85 years or more were admitted to hospital with a diagnosis of NSTE-ACS during the period of analysis: 182 (79.8%) with non-ST elevation acute myocardial infarction, 10 patients (4.4%) with ST depression but without troponin elevation and 36 (15.8%) with chest pain suggestive of unstable angina without ST segment depression or elevation of markers. A test for ischemia was performed in 9 patients (8 with exercise testing and 1 with stress echocardiography), which was positive in 3 patients. These 3 patients and 2 with a negative stress test were referred for catheterization. Echocardiography was performed on admission in 189 (82.9%) patients (78.1% of patients in the CM group and 89.0% in the IA group, P=.04).
According to the GRACE score, 189 patients (82.9%) were at high risk, 34 (14.9%) at intermediate risk and 5 patients (2.2%) at low risk; 4 of the latter had recurrent angina during hospitalization. Therefore, 227 patients (99.6%) in our study were indicated for catheterization according to the clinical practice guidelines.6
Any decision to perform coronary angiography was taken by the physician responsible for each patient. A total of 100 patients were chosen for IA and 128 for CM.
Patients who were treated invasively were younger and more often male, with a higher prevalence of dyslipidemia, history of heart failure, AMI and revascularization. Patients managed conservatively had more frequent atrial fibrillation, a higher prevalence of dementia and greater comorbidity. At admission, the invasively managed patients had a lower risk profile, with less presence of positive troponin and a lower Killip class, although there were no differences in the ejection fraction or the proportion of patients at risk according to the GRACE score. Patients with recurrent ischemia during hospitalization underwent IA more often. Table 1 shows the differences in baseline characteristics between the two groups.
As time passed, the decision to send patients for a coronary angiography became increasingly common. Before 1 January 2007, 40.6% of patients were referred for IA, and afterwards the figure rose up to 67% (P<.001). We also found this difference in matched patients (36.5% vs 73.0%, P<.001).
As described in Table 1, after matching patients by PS, the groups were well balanced, with no standardized difference >10% being found.
Coronary AnalysisThe angiographic and intervention features are described in Table 2.
Table 2. Angiographic and Intervention Features
| Angiographic features | |||
| No. diseased vessels (%) | Distribution of diseased vessels (%) | ||
| 0 | 3 | Left common trunk | 10 |
| 1 | 23 | Anterior descending | 73 |
| 2 | 33 | Circumflex artery | 69 |
| 3 | 41 | Right coronary artery | 63 |
| Procedure features | |||
| Procedure | Stents | ||
| Radial access (%) | 74 | No. stents/patient | 2 (1-7) |
| Contrast dose (mL) * | 193 (75-479) | Drug-eluting stents (%) | 24 |
| Ostial/bifurcation PCI (%) | 23 | Length stent/patient (mm), mean (range) | 35 (9-102) |
| Rotablation/cutting balloon (%) | 9 | Stent in LCT (%) | 8 |
LCT: left common trunk; PCI: percutaneous coronary intervention.
* Iodixanol (Visipaque®).
No percutaneous revascularization was performed in 32% of patients. The reasons were the presence of diffuse disease, thin capillaries or non-revascularizable disease in 65.4%, and the absence of severe lesions in 34.6% (9 patients). Revascularization was chosen for only one patient (who, in fact, died before surgery could be performed).
Percutaneous revascularization was performed in 68% of the patients, and a stent was implanted in all of them. Revascularization was successful in 94% of cases and complete in 40%.
Medical Treatment During HospitalizationTable 3 shows the treatment received during hospitalization. Patients who underwent coronary angiography received more oral antiplatelet agents, both aspirin and clopidogrel, and more statins, with no difference in other medication recommended by clinical practice guidelines. Only 6% of patients in the IA group and none in the CM group received glycoprotein IIb/IIIa inhibitors.
Table 3. Hospital Treatment in the Overall and Matched Samples for Conservative Management; and Interventional Approach
| Total sample | Matched sample | |||||
| CMN=128 | IAN=100 | P | CMN=63 | IAN=63 | P | |
| ASA | 93/114 (81.6) | 98/100 (98) | .001 | 51/58 (87.9) | 62/63 (98.4) | .02 |
| Clopidogrel | 26/114 (22.8) | 82/100 (82) | <.001 | 9/58 (15.5) | 51/63 (80.9) | <.001 |
| ASA+Clopidogrel | 16/114 (14) | 80/100 (80) | <.001 | 6/58 (10.3) | 50/63 (79.3) | <.001 |
| GPIIb/IIIa inhibitor | 0/114 (0) | 6/100 (6) | .004 | 0/58 (0) | 1/63 (1.6) | .31 |
| LMWH | 113/114 (99) | 100/100 (100) | .90 | 58/58 (100) | 63/63 (100) | 1 |
| Beta-blockers | 51/114 (44.7) | 53/100 (53) | .22 | 34/58 (58.6) | 29/63 (46.0) | .19 |
| Calcium antagonists | 38/114 (33.3) | 28/100 (28) | .52 | 23/58 (39.6) | 17/63 (27.0) | .13 |
| ACEI/ARB-II | 72/114 (63.2) | 64/100 (64) | .91 | 31/58 (53.5) | 32/63 (50.8) | .84 |
| Loop diuretics | 56/114 (49.1) | 35/100 (35) | .36 | 21/58 (36.2) | 26/63 (41.3) | .59 |
| Spironolactone | 9/114 (7.9) | 12/100 (12) | .17 | 4/58 (6.9) | 6/63 (9.5) | .56 |
| Nitrates | 84/114 (73.7) | 59/100 (59) | .24 | 42/58 (72.4) | 38/63 (60.3) | .17 |
| Statins | 55/114 (48.2) | 68/100 (68) | .001 | 31/58 (53.4) | 37/63 (58.7) | .54 |
| Acenocumarol | 12/114 (10.5) | 10/100 (10) | .96 | 6/58 (10.3) | 7/63 (11.1) | .84 |
| Antiarrhythmic agents | 9/114 (7.9) | 11/100 (11) | .36 | 4/58 (6.9) | 7/63 (11.1) | .39 |
| Digoxin | 9/114 (7.9) | 8/100 (8) | .95 | 5/58 (8.6) | 5/63 (7.9) | .93 |
| Iron | 20/114 (17.5) | 12/100 (12) | .29 | 8/58 (13.8) | 7/63 (11.1) | .7 |
ACEI, angiotensin-converting enzyme blockers; ARB-II, angiotensin receptor blockers II; ASA, acetylsalicylic acid; CM, conservative management; GPIIb/IIIa, glycoprotein IIb/IIIa; IA, interventional approach; LMWH, low molecular weight heparins.
Results are expressed as n/N (%).
Hospital events are shown in Table 4.
Table 4. Hospital Development in the Overall and Matched Samples for Conservative Management; and Interventional Approach
| Total sample | Matched sample | |||||
| CMN=128 | IAN=100 | P | CMN=63 | IAN=63 | P | |
| TIMI bleeding | 8/114 (7) | 8/89 (9) | .62 | 5/58 (8.6) | 5/56 (8.9) | .93 |
| Transfusion | 10/126 (7.9) | 8/96 (8.3) | .91 | 6/62 (9.7) | 6/62 (9.7) | 1 |
| Renal function deterioration | 7/105 (6.7) | 11/95 (11.6) | .22 | 3/50 (6) | 7/56 (12.5) | .22 |
| Stroke | 4/128 (3.1) | 1/98 (1) | .39 | 3/63 (4.8) | 1/62 (1.6) | .31 |
| ReAMI | 0/128 (0) | 4/98 (4.1) | .03 | 0/63 (0) | 1/62 (1.6) | .31 |
| Death | 15/128 (11.7) | 5/100 (5) | .08 | 5/63 (7.9) | 2/63 (3.2) | .25 |
CM, conservative management; IA, interventional approach; ReAMI, readmission due to acute myocardial infarction.
Results are expressed as n/N (%).
IA group patients had more non-fatal reinfarctions. Two patients had sub-acute stent thrombosis. 4% of those undergoing angiography had a complication during the procedure (3 coronary dissections, 1 artery rupture without tamponade), and 7% vascular complications (3 radial perforations, 3 femoral hematomas and 1 femoral pseudoaneurysm). None of them required surgery.
There was no major bleeding and no difference in the occurrence of minor bleeding or transfusion requirements between the two groups.
In the PS matching, there were no differences in the incidence of hospital events analyzed (Table 4).
Events During MonitoringFollow-up at 1, 2 and 3 years was performed in 98.2%, 90.8% and 78.9% of the patients, respectively. Overall events in the study (not adjusted for PS) are shown in Table 5.
Table 5. Events during the 3-years Follow-up in the Overall and Matched Samples for Conservative Management; and Interventional Approach
| Total sample | Matched sample | |||||
| CMN=128 | IAN=100 | P | CMN=63 | IAN=63 | P | |
| Death | 79/128 (61.7) | 31/98 (31.6) | <.001 | 33/63 (52.4) | 21/63 (33.3) | .037 |
| Death or MACE | 92/128 (71.9) | 43/98 (43.9) | <.001 | 43/63 (68.3) | 26/63 (41.3) | .003 |
| Readmission - AMI | 25/128 (19.5) | 11/98 (11.2) | .08 | 16/63 (25.4) | 7/63 (11.1) | .038 |
| Readmission - angina | 9/128 (7.0) | 9/98 (9.2) | .58 | 7/63 (11.1) | 5/63 (7.9) | .54 |
| Readmission - stroke | 8/128 (6.3) | 4/98 (4.1) | .45 | 4/63 (6.4) | 4/63 (6.4) | 1 |
| Readmission - bleeding | 10/128 (7.8) | 10/98 (1.2) | .56 | 4/63 (6.4) | 5/63 (7.9) | 1 |
| Readmission - CHF | 30/128 (23.4) | 24/98 (24.5) | .92 | 14/63 (22.2) | 18/63 (28.6) | .41 |
AMI, acute myocardial infarction; CHF, congestive heart failure; CM, conservative management; IA, interventional approach; MACE, major adverse cardiovascular events.
Results are expressed as n/N (%).
The relationship between coronary angiography and events (death and death or MACE) adjusted for PS quintiles and the year of admission are shown in Table 6. It can be seen that, adjusting for PS quintiles, coronary angiography in the whole study is associated with lower mortality (HR 0.518; 95% CI, 0.316-0.850) and fewer deaths or MACE at follow-up (HR 0.483; 95% CI, 0.315-0.741). This result does not change when adjusted for year of admission (mortality HR 0.465, 95% CI, 0.279-0.775 and death or MACE: HR 0.459; 95% CI, 0.294-0.715). The relationship between PS quintiles and mortality in this multivariate analysis approaches significance (P=.1).
Table 6. Cox Regression for Interventional Approach Adjusted for Propensity Score Quintiles and Matching
| Wald | P | Hazard Ratio | 95% CI OR | |
| Hazard ratio of interventional approach adjusted for propensity score quintiles | ||||
| Death | ||||
| Propensity score quintiles | 7.71 | .103 | ||
| Interventional approach | 6.789 | .009 | 0.518 | 0.316-0.85 |
| Death or MACE | ||||
| Propensity score quintiles | 4.917 | .296 | ||
| Interventional approach | 11.121 | .001 | 0.483 | 0.315-0.741 |
| Hazard ratio of interventional approach adjusted for propensity score quintiles and year of admission (2004-2006 vs 2007-2009) | ||||
| Death | ||||
| Propensity score quintiles | 7.317 | .12 | ||
| Interventional approach | 8.627 | .003 | 0.465 | 0.279-0.775 |
| Year of admission | 2.395 | .122 | ||
| Death or MACE | ||||
| Propensity score quintiles | 5.183 | .269 | ||
| Interventional approach | 11.834 | .001 | 0.459 | 0.294-0.715 |
| Year of admission | 0.805 | .37 | ||
| Hazard ratio of interventional approach in the matched sample | ||||
| Death | ||||
| Interventional approach | 4.125 | .042 | 0.567 | 0.328-0.98 |
| Death or MACE | ||||
| Interventional approach | 8.275 | .004 | 0.489 | 0.300-0.796 |
| Hazard ratio of interventional approach in the matched sample adjusted for year of admission (2004-2006 vs 2007-2009) | ||||
| Death | ||||
| Interventional approach | 5.194 | .023 | 0.502 | 0.277-0.908 |
| Year of admission | 1.101 | .294 | ||
| Death or MACE | ||||
| Interventional approach | 8.542 | .003 | 0.454 | 0.267-0.771 |
| Year of admission | 0.589 | .443 | ||
CI, confidence interval; MACE, major adverse cardiovascular events; OR, odds ratio.
We analyzed the 3-year prognosis of 126 PS-matched patients, whose characteristics are shown in Table 1.
There were 2 deaths during hospitalization in the IA group and 5 in the CM group. The survival analysis included events during hospitalization.
Follow-up at 1, 2 and 3 years was performed in 99.2%, 88.9% and 75.4% of the patients, respectively. The median follow-up was 151 weeks (interquartile range: 110-182 weeks) without differences between the groups (median: 154±59 vs 150±48, P=.78).
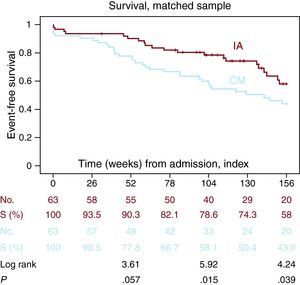
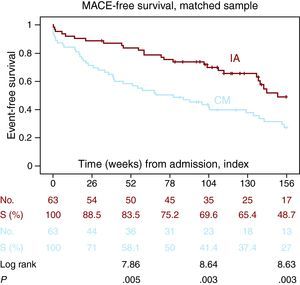
During this period, patients managed invasively had increased survival at 2 years (78.6% vs 58.1%, P=.015) and 3 years (58% vs 43.9%, P=.039) (Figure 2). There was also a higher combined MACE-free survival (83.5% vs 58.1%, P=.005) at 2 years (69.6% vs 41.4%, P=.003) and 3 years (48.7% vs 27%, P=.003) (Figure 3).
Figure 2. Probability of survival according to coronary angiography (Kaplan-Meier). IA, interventional approach; CM, conservative management.
Figure 3. Probability of survival without major adverse cardiovascular events according to coronary angiography (Kaplan-Meier). IA, interventional approach; CM, conservative management; MACE, major acute cardiovascular events.
There were no significant differences in readmission due to bleeding or heart failure (Table 5).
DISCUSSIONThe main findings of this study are that in unselected patients of a very advanced age with high comorbidity, coronary angiography during hospitalization with the intention of revascularization in NSTE-ACS is associated with increased survival and MACE-free survival at three years, with a low rate of procedure-related complications and no significant increase in bleeding.
A previous study found the same results in slightly younger patients (mean age of 80.4 years).19 However, we found no studies in patients so old or with variables related to comorbidity. We included the Charlson index which has a prognostic value demonstrated in patients admitted with ACS.20
The analysis of our cohort study confirms that very elderly patients are a special high risk subgroup in the context of NSTE-ACS. In our series, 97.8% of patients were at moderate or high risk according to the GRACE score. The treatment recommended by clinical practice guidelines for these patients is to perform a coronary angiography.6
In different records on the management of NSTE-ACS in both Spain (MÁSCARA21) and other countries (CRUSADE,22 GRACE2) a tendency to select intervention management for patients with a lower risk profile has been described. This is also reflected in our cohort study of very elderly patients. Those receiving IA had lower GRACE scores, lower percentage of positive troponin and lower Killip class.
In our series, a bias was introduced by the doctor treating the patient, with a tendency to decide CM in elderly patients with comorbidity; which has also been reported in other series.23, 24 These patients also received less of other actions recommended by clinical practice guidelines: less dual antiplatelet therapy and statins and fewer echocardiographies during hospitalization.
One of the possible reasons for not performing IA in elderly patients is the perception that the risk of complications exceeds the benefit of the revascularisation.22 In our study, only 4% of patients suffered from complications during the procedure, which is comparable to the rate described for other population groups.25 Moreover, the rate of bleeding or need for transfusion during hospitalization was low compared with other series,21, 26 and did not differ significantly between patients managed conservatively or invasively. This may be due to the scarce use of the glycoprotein IIb/IIIa inhibitor in our series, and because most procedures were performed via the radial approach, which has been proven to minimize the risk of bleeding.27 We also found no more readmissions due to bleeding in the follow-up, despite receiving a greater proportion of dual antiplatelet therapy.
Although the radial approach was frequently used, the rate of vascular complications in our series was higher than that reported in other series of younger patients.28 This is expected, due to the increased arterial calcification and tortuosity in older patients. None of the complications required surgical intervention.
In our series, 68% of patients referred for coronary angiography underwent revascularization, which is similar to the percentage described in randomized trials and other studies in younger patients.19, 26
LimitationsThe main limitation of our study was the small number of patients, especially after matching for PS, which may limit the ability to generalize. This is because we chose very elderly patients operated upon in a single centre. However, despite the small sample, significant differences were found in the medium-term follow-up studies, similar to those of studies in younger patients.
The treatment groups are very different due to an important selection bias. An adjustment using PS was made to minimize such bias, but it may not have been completely eliminated. This is because we used it in a small sample, and because unrecorded variables may have led to bias. After matching, we compared the baseline characteristics of the groups to make sure the differences had disappeared. However, given that it was a retrospective study, it was not possible to record variables shown to influence prognosis in the elderly population with ischemic heart disease, such as the frailty index, capacity for self-care or institutionalization.29 These variables may be distributed differently between the two groups, leading to a bias in the results.
Another limitation was the increasing tendency to perform coronary angiography as the study progressed. There may have been advances in treatment that favour intervention (better training of cardiologists, change of access route, better devices or new drugs). However, we adjusted for year of admission using the Cox regression model without changing the relationship between coronary angiography and prognosis.
IA group patients received clopidogrel and dual antiplatelet therapy much more frequently, and this may have influenced the better results for this group.
CONCLUSIONSIn our series, almost all very elderly patients admitted with NSTE-ACS were at moderate or high risk, and as such, current clinical practice guidelines would have indicated a coronary angiography.
Our data suggest that this management offers a better prognosis in the medium term, with a low rate of procedural complications and no significant increase in the rate of bleeding.
CONFLICTS OF INTERESTNone declared.
Received 2 December 2010
Accepted 19 April 2011
Corresponding author: Donibane Garazi 4, 3C, 20018 San Sebastián, Guipuzcoa, Spain. inaki.villanuevabenito@osakidetza.es