Keywords
*A list of MASCARA study participating researchers and hospitals can be found at the end of the article.
INTRODUCTION
Peripheral arterial disease (PAD) and cerebrovascular disease (CVD) have repeatedly been identified as markers of increased cardiovas cular risk in several types of population, including the general population, those at risk of cardiovascular disease, patients with a history of cardiovascular events1 or with a low anklebrachial index.2
Several studies have shown that short- and medium-term prognosis in coronary heart disease is worse when other vascular beds are also affected (polyvascular disease [PD]). However, most of these studies were sub-analyses of clinical trials3-7 or studies conducted in selected populations undergoing cardiac catheterization,8-11 revascularization surgery,12 or both.13 In unselected patients admitted for acute coronary syndrome (ACS), 2 studies have confirmed that in-hospital14 and 6-month outcomes15 are poorer when PAD is present. Those studies also indicated that some of the evidence-based treatments recommended for ACS may be under-utilized in these patients. To our knowledge, only one study has analyzed the prognostic value of concomitant PAD and CVD in unselected patients admitted for ACS.15 Furthermore, the definition of CVD in that study was limited to patients with antecedents of cerebral ischemia or transient ictus.15 The precise role of PAD and CVD, or both, in patients with ACS is currently unclear and real-world treatment patterns in this group have been not been sufficiently studied.
In this prospective cohort study we examined the clinical profile of patients with PAD, CVD, or both and analyzed the independent prognostic value of these conditions on in-hospital and 6-month outcomes. We also studied patterns of care in a population of patients with ACS under conditions of current clinical practice. As patients were drawn from a national ACS registry, the results are applicable to Spain as a whole. This makes them of particular interest as Spain has a significantly lower cardiovascular mortality rate than that of other, similar regions.
METHODS
A complete description of the MASCARA registry (Managing Acute Coronary Syndrome: current registry) has been published previously.16,17 In short, MASCARA is a prospective, multicenter registry applied in 32 randomly selected Spanish hospitals which met inclusion requirements.
Study Population
Patients were included if a diagnosis of ACS had been confirmed by any of the following criteria:
a) markers of myocardial damage above the normal upper limit for the referring laboratory;
b) movement of ST segment =1 mm; c) stress test during admission indicating myocardial ischemia; and d) known coronary artery disease. The only exclusion criteria were: a) non-cardiac disease with expected survival of less than 1 year; b) ischemia not caused by heart problems; and c) impossibility of follow-up. The need for consecutive inclusion of patients was stressed to participating centers. The study was approved by the Ethics Committees of the participating institutions.
At each site, the physician responsible for the study or a coordinator identified patients who met inclusion and exclusion criteria, requested their informed consent to participate, and classified them as ACS with ST elevation, non-ST elevation ACS, or indeterminate ACS based on electrocardiogram (ECG) results on admission. Trained external investigators later recorded demographic and clinical variables, treatments, and in-hospital outcomes on standardized forms.
For the present study, patients with data indicating PAD, CVD or both were identified. A patient was considered to have PAD when there was a clinical history of intermittent claudication, when they had undergome peripheral arterial surgery or angioplasty, or when amputation had been performed because of arterial disease. Cerebrovascular disease was considered to be present when there was a history of stroke or transient cerebral ischemia or when imaging studies indicated carotid or vertebrobasilar stenosis. Cerebrovascular disease was not considered present in patients with a history of stroke or transient cerebral ischemia who had received a prosthetic valve or who were suffering from atrial fibrillation unless prior imaging studies demonstrated significant stenosis in the territories in question. Study outcomes were in-hospital death, stroke and major hemorrhage or death at 6 months. All relevant events and characteristics were identified through predefined variables used in the MASCARA study protocol.16, 17
Follow-up
Patient follow-up was by telephone interview 1 and 6 months after discharge. All telephone calls were centralized and were carried out by trained interviewers.
Statistical Analysis
Continuous variables are presented as means and standard deviations or medians (interquartile ranges) as appropriate, and categorical data are presented as percentages.
We compared baseline variables, procedures, treatments, and clinical outcomes among patients without any type of arterial disease (PAD, CVD, or both) and those with any of the conditions. Categorical variables were compared using the chi-squared test and Student t test was used for continuous variables. Comparisons between patients with PAD, CVD or both were performed using Fisher exact test for categorical variables and ANOVA for continuous variables.
The independent association between PAD, CVD, or both and clinical outcomes was explored using multivariate non-conditional logistic regression models. We selected potentially predictive variables based on clinical plausibility and their association with clinical outcomes observed in univariate analysis (all variables significant at P<.2 were selected). First, in stepwise fashion we established factors associated with an outcome. The resulting models were then controlled using a standardized list of factors. Selected variables were age, sex, cardiovascular risk factors (smoking, high blood pressure, diabetes, hypercholesterolemia), comorbidities (renal failure, prior myocardial infarction, prior percutaneous interventions, previous coronary surgery, history of heart failure), characteristics at admission (type of ACS, positive markers of necrosis, Killip grade, heart rate, systolic blood pressure), pharmacological treatment within 48 hours (aspirin, low molecular weight heparin, thienopyridines, glycoprotein IIb/IIIa inhibitors, beta-blockers), intrahospital management (percutaneous intervention, coronary artery surgery) and therapeutic prescription at discharge (aspirin, thienopyridines, beta-blockers, angiotensin converting enzyme [ACE] inhibitors, statins, diuretics ). We also analyzed first-order interactions between covariates.
Cumulative survival was assessed using the Kaplan-Meier method. Survival curves were compared using the log-rank test. All P-values are two-tailed and values of P<.05 were considered significant.
RESULTS
In total, the MASCARA registry included 7251 patients, of which 506 (7%) were not included in the present study due to incomplete data. A total of 6745 patients were therefore included for analysis. Of these, 597 (8.8%) had PAD, 392 (5.8%) had CVD, 131 (1.94%) had both PAD and CVD, and 5625 (83.4%) did not have either condition. Table 1 shows the demographic characteristics, cardiovascular risk factors, prior history, and medications in the 4 patient subgroups. The PAD patients were older, predominantly male, and more frequently had a history of myocardial infarction, treatment with antiplatelets, and aortocoronary grafts. Non-ST elevation ACS or indeterminate ACS was more frequent in PAD patients, as shown in Table 2. Despite a higher proportion of patients with Killiip grades III and IV, positive markers of myocardial injury, and elevated serum creatinine in the first 48 h after admission, patients with PAD were less frequently treated with aspirin, glycoprotein IIb/IIIa inhibitors, and beta-blockers, and received fewer coronary angiographs. Early hospital mortality in these patients was also higher. During the hospital stay, echocardiograms, stress tests, and coronary angiographs were less frequently performed in patients with PAD (Table 3). Among patients in whom coronary angiography was performed (approximately 60%), coronary artery disease was more extensive in those with PD who showed higher rates of 3 vessel and common trunk disease. Percutaneous interventions were also performed less frequently during hospital stay in patients with PAD and CVD than in those without the disease, but the lowest rate of percutaneous intervention was in patients with concomitant PAD and CVD. The in-hospital mortality rate was almost double in patients with PAD or CVD (9.1% and 9.2%, respectively) compared to those without either disease (4.8%), though in patients with concomitant PAD and CVD mortality was almost 4 times higher (16%). In-hospital rates of ischemic stroke and severe bleeding were also significantly higher in patients with PD.
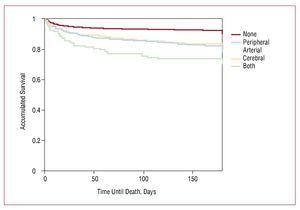
At discharge, aspirin, beta-blockers, and statins were clearly prescribed less frequently to patients with PD than to the other patients (Table 4). Among PD patients, those with 2 artery disease were treated with thienopyridines significantly less frequently than patients with only single artery non-coronary disease. By contrast, a higher proportion of patients with PD received diuretics and oral anticoagulants. Figure shows survival at 6 months, with a clear stratification between patients who were free of associated arterial disease and those with PAD, CVD, or both. In general, non-coronary arterial disease was not independently associated with in-hospital and 6-month mortality (Tables 5 and 6). This was particularly true in PAD patients and those with both diseases. When only the 6358 patients who survived hospitalization were analyzed, PAD and CVD remained independent predictors of mortality at 6 months (odds ratio [OR] =1.78; 95% confidence interval [CI], 1.19-2.6 and OR=1.95; 95% CI, 1.41-2.69, respectively), but in the group with PAD and concurrent CVD the difference was no longer significant, although the number of patients surviving hospitalization in that group was low.
Figure. Survival from the index episode according to the presence and site of arterial disease
DISCUSSION
This study shows that patients in clinical practice with ACS and PD have, in general, more extensive and severe coronary disease and poorer clinical outcomes at 6 months. Moreover, in this study, therapeutic interventions that are recommended as effective in clinical practice guidelines were used less extensively in patients with ACS and PD than in ACS patients without concomitant coronary artery disease. The clinical significance of PD in patients with atherosclerosis is increasingly recognized. First, it has been observed in a substantial proportion of patients from various populations, including community-based samples,1 patients referred for hospital care,2 patients in clinical trials,7 or those with ACS.15 The frequency of non-coronary artery disease in these populations varied between 8.1%7 and 43%.15 In our study, the frequency of PD was around 17%, which was lower than the rate observed by Mukherjee et al,15 in patients with ACS. This difference could be explained by the different prevalence of atherosclerotic disease profiles in the two studies. In all of these studies, however, PD occurred with considerable frequency.
Second, PD has been shown to predict cardiovascular events in all of these populations. In the REACH registry, cumulative risk of major cardiovascular events at 1 year was observed in apparently stable outpatients. Increased risk ranged from 5.3% in patients with only one risk factor to 26.3% in patients with three arterial disorders, giving an almost linear increase.1 In a recent study,15 PD in patients with ACS was associated with poor clinical outcomes. Our study showed that in patients with ACS, presence of PD was independently associated with in-hospital and 6-month mortality. The finding was particularly consistent in patients with PAD.18 The association with in-hospital mortality was also particularly constant in patients with both types of arterial disease. Even when patients who died during the index episode were excluded from the analysis, PAD and CVD remained independent predictors of mortality, although this was not true in patients with the 2 types of disease, most likely due to the small number of patients in this subgroup and because mortality occurred most often during the initial period. In patients suffering only from PAD or CVD, in-hospital mortality and 6-month risk was as high as in diabetes patients or those with positive necrosis markers. The prognostic impact of PAD and CVD and their relevance to clinical decision-making in patients with ACS have probably not yet been adequately appreciated amongst clinicians.
In this sense, it is interesting to note that PAD is not one of the variables used in instruments to evaluate risk in ACS patients, such as the GRACE, TIMI, or PURSUIT scores.19 It is likely that PAD and CVD were not included in these systems because they were seen as only marginally increasing their discriminative capacity. It is well established that comorbidity as a whole is an important negative factor in prognosis, as has been shown in studies which adjusted their results using the Charlson index.20 In any case, the clinical message of our findings is clear: all other prognostic factors being equal, ACS patients with concomitant PAD or CVD should be considered as having a poorer clinical prognosis, particularly when both PAD and CVD are present, and this should be taken into account in their management. Poor prognosis in these patients may stem in part from the presence of more extensive and severe coronary disease as well as from less than optimal treatment. Our PAD patients more often had multivessel and left main disease, though these findings have yet to be analyzed in depth. All of these observations indicate that, rather than thinking of atherosclerosis at non-coronary sites as associated disease, it is preferable to think of vascular patients as suffering from a single process whose extension has a clear influence on prognosis. The idea that atherosclerosis should be considered in practice as an integral disease rather than the sum of a set of independent diseases was highlighted in the REACH registry, which showed that overall risk did not depend on the site of initial arterial disease, but on its extension.1
Given the finding of greater severity and worse prognosis in patients with ACS and PD it may seem surprising that these patients receive less intensive treatment. Glycoprotein IIb/IIIa inhibitors, beta blockers, early coronary angiography, and other evidence-based treatments were less frequently used in these patients, both during hospitalization and at discharge. On the other hand, Mukherjee's study showed that PAD patients who received evidence based treatment had better clinical outcomes and that prognosis was better the higher the number of interventions performed. Furthermore, clinical practice guidelines clearly recommend more vigorous treatment in patients at greatest risk.
It is unclear why then, in this unselected sample of hospitals from around Spain, patients with PD received less treatment. Similar recent findings were observed in registries from different geographical areas which indicated that low-risk ACS patients without ST-segment elevation were more likely to receive early interventional treatment.21,22 Less frequent early interventional strategies were also observed in patients with PD in the study by Mukherjee et al.15 The reasons for this are speculative and presumably complex. Higher risk is often associated with greater vulnerability. Clinicians may reasonably judge patients at greater risk to be too frail for invasive treatment, though it is difficult to measure this objectively. Clinicians may also refrain from using drugs in these patients due to an increased fear of side effects or the existence of objective contraindications. For example, although early treatment with beta-blockers reduces inhospital and 6-month mortality, they are often withdrawn in patients with PAD due to the risk of reducing blood flow to the lower extremities. However, in some cases a culturally-based fatalistic attitude or excessive prudence may also come into play. Whatever the explanation, the finding deserves further attention.
Study limitations, as with any registry based study, include the possibility of biases, though these would be minimized by the random selection of participating centers and by the use of consecutive inclusion. It is unlikely that the number of patients excluded because of incomplete data would introduce significant bias because, in another MASCARA cohort study, we found that the characteristics of patients with incomplete data were similar to those of patients with complete information.22 Likewise, although we analyzed the relationship between use of treatments and clinical outcomes after adjusting for various potentially confounding demographic and clinical characteristics there may still be some residual confounding from other factors and the therapeutic indication. Furthermore, the diagnosis of arterial disease was not likely to be highly sensitive, given the low sensitivity of the usual clinical methods for diagnosis. These would also be further magnified in a study with a retrospective design. On the other hand, it should be noted that, as in other studies1 that recorded only symptomatic arterial disease, the degree and risk of PD in this study would be understated. Therefore, our findings indicate the serious prognostic significance of concomitant arterial disease in patients with ACS and highlight the need to take it into account.
CONCLUSIONS
Patients with ACS and PD observed in clinical practice have more extensive and more severe coronary disease and worse clinical outcomes at 6 months. Despite that, therapeutic interventions recommended as effective in clinical practice guidelines are typically used less extensively in these patients. Polyvascular disease should be regarded as a single process whose extension has a clear influence on prognosis.
MASCARA Study Investigators
Dr Radován and Dra Maulén (H. de Campdevanol; Gi rona). Dr Ortiz de Murúa, Dr Marcos, and Dr Arribas (H. Virgen de la Concha; Zamora). Dr Laperal and Dr Casado (H. de Calatayud; Zaragoza). Dr Bisbe (H. Sant Jaume de Olot; Girona). Dr Bartomeu, Dra Carrillo and Asunción Mateu (H. Universitario Sant Joan d'Alacant). Dr Gutiérrez and Dr. Benítez (H. Virgen del Puerto; Plasencia). Dr de Miguel, Dra Martínez, and Dra. Soriano (H. de Terrassa). Dr Arias and Isabel Gómez (H. de Montecelo; Pontevedra). Dr Ortega and Dr Molina (H. Sta María del Rossell; Cartagena). Dr Herreros and Dr Azcárate (Clí nica Universitaria de Navara). Dr Worner, Dr Piqué, and Purificación Cascant (H. Arnau de Vilanova; Lérida). Dr Salvador and Dr Aguar (Clínica Dr Pesset; Valencia). Dr Arós and Dr Sanz (H. de Txagorritxu; Vitoria). Dr Velasco and Dra Belchi (H. Gral. Universitario de Valencia). Dr Pagola and M Amparo Pérez (H. Ciudad de Jaén). Dr Sogorb and Dra Oliver (H. Gral. Universitario de Alicante). Teresa Martorell, Dr Bórqued, and Dr Verbal (H. Clínic i Provincial; Barcelona). Dr Esplugues, Dr Ribas, and Cristina Carvajal (Ciudad Sanitaria de Bellvitge; L´Hospitalet de Llobreregat). Dr Martín and Dr Pabón (H. Universitario de Salamanca). Dr Froufe, Dra León, and Dr. Montes (H. de Cruces; Baracaldo). Dr Poveda, Dra Ruiz, and Marta Calvo (H. Universitario Marqués de Valdecilla; Santander). Dr Alcalde, Dr Alguersuari, Dr Otaegui, and Purificación Cascant (H. Vall d'Hebron; Barcelona). Dr Juan, Dra Barrio, and Dr Estévez (H. Universitario Gregorio Marañón; Madrid). Dr Moreno and Dra Martín (H. San Cecilio; Granada). Dr Fernández Avilés and Dr Sánchez (H. Clínico Universitario de Valladolid). Dr Bruguera, Dra Soriano, and Dr Recasens (H. del Mar; Barcelona). Dr Abizanda and Dra Micó (H. Gral. de Castellón). Dra Huelmos (Fundación Hospital de Alcorcón; Madrid). Dr Ortigosa and Dr Silva (Clínica Puerta de Hierro; Madrid). Dr Bardají, Dra Serrano, and Purificación Cascant (H. Joan XXIII; Tarragona). Dr Sala, Isabel Ramiò, and Ruth Martí (H. Josep Trueta; Girona). Dr Montón (H. Gral. Yagüe; Burgos). Dr Casares and Dr. Blanco (H.S. Agustín de Avilés). Dr Calvo Iglesias and Dr O. Díaz (H. Meixoeiro; Vigo). Dr Munilla and Dr A. Marquina (C.H. San Millán S. Pedro; La Rioja). Dr F. Noriega and Dra M. Vázquez (Policlínico de Vigo). Dr Valdepeñas and Dra Montero (H. de Alarcos; Ciudad Real). Dr Torres, Dr Lesmes, and Dra Melguizo (C.H. Ntra. Sra. de Valme; Sevilla). Dr Aguirre and Dra M. Lluis (H. de Basurto). Dr Llamas, Dra Iriondo, and Dr. Arrate (H. Ntra. Sra. Aránzazu; San Sebástiá). Dr de Teresa, Dr Jiménez, and Dr A.I. Pérez (C.H. Virgen Victoria; Málaga). Dr R. Padial and Dr Corrochano (H. Virgen de la Salud; Toledo). Dr Merchán (C.U. Infanta Cristina; Badajoz). Dr Monzón, Dr Sánchez, and Dr Chabbar (H. Miguel Servet; Zaragoza). Dr Calvo, Dr Cruz, and Dr González (H. Virgen Macarena; Sevilla). Dr Amador, Dra Durán, and Dra. Rodríguez (C.H. Reina Sofía; Córdoba). Dr Hernando and Dr. Macaya (C.U. San Carlos; Madrid). Dra Cabezón and Dra Hernández (C.H. Virgen del Rocío; Sevilla). Dr Lecuona and Dra Morillas (H. Galdakao). Dr Romero (Fundación Jiménez Díaz; Madrid).
ABBREVIATIONS
ACS: acute syndrome coronary
CVD: cerebrovascular disease
PAD: peripheral arterial disease
PD: polyvascular disease
This study was supported by grants from the Fondo de Investigación Sanitaria (PI04/1408), the Red de Investigación Cardiovascular del Instituto Carlos III (RECAVA), and by an unrestricted grant from Bristol-Myers-Squibb.
Correspondence: Dr. G. Permanyer-Miralda.
Unidad de Epidemiología. Servicio de Cardiología. Hospital Vall d'Hebron. Pg. Vall d'Hebron, 119-129. 08035 Barcelona. España.
E-mail: gpermany@gmail.com
Received March 20, 2009.
Accepted for publication May 20, 2009.