In 1944, Alfred Blalock, a vascular surgeon from Baltimore, following the theoretical instructions of Helen B. Taussig, a pediatric cardiologist from the same city, connected the left subclavian artery to the ipsilateral pulmonary artery in a cyanotic patient with tetralogy of Fallot (TOF). In 1955, John W. Kirklin performed the first complete surgical repair of this defect with the use of a cardiopulmonary bypass machine. Since then, the clinical outcome of this disease has changed dramatically. While the first studies on the natural history, published by Maurice Campbell 50 years ago, showed that most patients died as children, nowadays, more than 90% of patients with repaired TOF (rTOF) survive to adult age and this cardiac malformation is now the most prevalent congenital heart disease in adults in Spain.1
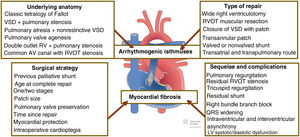
However, the age- and sex-adjusted life expectancy for adults with rTOF remains lower than that of the general population.2 The excess mortality is related to the risk of sudden cardiac death (SCD) during the first decades of life and the onset of heart failure in more advanced years.3 This means that one of the biggest dilemmas in the clinical follow-up of this population is how to effectively prevent SCD. Although atrioventricular block, atrial tachycardia with rapid conduction, or certain mechanical complications such as acute pulmonary embolism have been linked with this clinical catastrophe, ventricular arrhythmias are, without doubt, the main cause. The pathogenesis of ventricular arrhythmias in rTOF is complex. Two key interactive mechanisms are the presence of corridors between scar areas, valve annuli, and postoperative patches, and diffuse myocardial fibrosis affecting the muscular mass of one or both ventricles.4 The first mechanism primarily relates to the underlying anatomy and the type of repair, and the second, to the surgical strategy and mechanical complications (figure 1).
Anatomic substrate, type of repair, surgical strategy, and mechanical complications in the pathogenesis of ventricular arrhythmias in repaired tetralogy of Fallot. Diagram adapted with permission from https://www.chd-diagrams.com. AV, atrioventricular; LV, left ventricle; RV, right ventricle; RVOT, right ventricular outflow tract; VSD, ventricular septal defect.
Classically, this defect is defined as a combination of 4 pathological anatomical elements: a) stenosis of the right ventricular (RV) outflow tract; b) a large, subaortic ventricular septal defect (VSD); c) an overriding aortic root; and d) LV hypertrophy. However, in a broader sense, the eponymous TOF usually includes any combination of nonrestrictive VSD and right ventricular outflow tract obstruction, independently of the location of the VSD or the stenosis. The aim of surgical repair is to release the outflow tract obstruction and close the VSD. Typically, this is done through a wide ventriculotomy incision, closure of the VSD using a synthetic patch, and widening of the outflow tract also with a patch that, as most patients have a hypoplastic pulmonary annulus, often involves transannular extension. So, between the VSD and outflow tract patches, the ventriculotomy incision, and the anatomical obstacles such as the tricuspid annulus and the pulmonary artery, there are a series of corridors that determine the preferred electrical conduction circuit. Often, these corridors have areas of slow conduction facilitating the macrore-entry mechanisms that lead to ventricular tachycardia (VT).5 However, intervention methods vary widely. Over time, the ventriculotomy incision has become smaller and, in many cases, has been replaced by a combined approach through the right atrium and pulmonary artery. In other cases, the obstruction is repaired through insertion of a valved (or nonvalved) shunt between the RV and the pulmonary artery that may even have an extra-anatomical arrangement. Therefore, the structural and functional spectrum and the electroanatomical substrate is widely heterogeneous, and this variability determines the surgical repair procedure and incidence of late complications, including ventricular arrhythmias and SCD.
Originally, surgical repair was delayed until the age of 6 to 12 months, and palliative interventions were needed in the interim to improve hypoxemia. The Blalock-Taussig, Waterston-Cooley, and Potts shunts were designed to increase pulmonary flow and reduce cyanosis. Although clinically effective, these shunts prolong hypoxemia and cause volume overload of the left ventricle (LV), resulting in diffuse myocardial ischemia. In the 1980s, advances in extracorporeal circulation with myocardial protection methods (cardioplegia) marked a watershed by reducing intraoperative myocardial ischemia and the incidence of permanent infarctions. Over the years, the timing of repair moved earlier toward the first 30 days of life, avoiding the need for palliative shunting. However, the small size of the neonatal heart required the use of a large patch for transannular extension, affecting the severity of pulmonary regurgitation (PR) and LV volume overload. More recently, to reduce the severity of PR, widening of the outflow tract is done using smaller patches, but this strategy can cause a higher persistent pressure gradient and greater pressure overload on the RV.
The different surgical strategies determine the mechanical sequelae that cause volume or pressure overload of one or both ventricles. The main mechanical sequela is PR, which is practically universal when transannular extension patches are used. An association was soon noted between the severity of PR and the incidence of sustained VT and SCD, and it became more popular to perform early pulmonary valve replacement to reduce the hemodynamic consequences of severe chronic PR. Unfortunately, it has still not been demonstrated that prosthetic replacement of the pulmonary valve reduces the incidence of ventricular arrhythmias or SCD, and the timing of pulmonary valve replacement in rTOF remains controversial. The main electrophysiological effect of rTOF is a widened QRS complex. The morphology of the complex usually shows a right bundle branch block pattern, often accompanied by end-QRS notching (fragmentation). The QRS widening and morphology has been attributed to RV dilatation and dysfunction and to the extent of myocardial scarring and intraventricular conduction delay. Together, the RV volume overload and the increased duration of the QRS complex alter the electrical and mechanical synchrony between the 2 ventricles, with adverse effects on the LV mechanics in both systole and diastole.
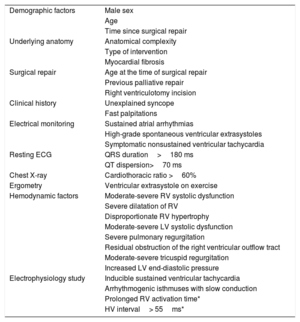
It is not surprising, then, that multiple predictive factors have been described in association with the incidence of ventricular arrhythmias and SCD in rTOF (table 1). These predictors include demographic, anatomical, surgical, clinical, electrocardiographic, radiological, ergometric, hemodynamic, and electrophysiological factors. However, individually, the positive predictive value of each of these predictors is rather limited. This is reflected in the North American7 and European8 recommendations for implantable cardioverter-defibrillator as primary prevention. As well as the class I recommendation, the same as for acquired heart disease (systemic LV ejection fraction<35%, biventricular physiology, and New York Heart Association functional class II-III), the guidelines have opted to underpin their recommendations with a IIa indication in those with multiple risk factors, such as systolic or diastolic LV dysfunction, nonsustained VT on Holter monitoring, QRS ≥ 180 ms, extensive scarring of the RV on cardiac magnetic resonance, or inducible VT on electrophysiology study. However, when the predictive value of these recommendations was analyzed in a large multicenter cohort of more than 25 000 adults with congenital heart disease and 171 cases of SCD secondary to arrhythmias, it was found that more than 60% of the cases would have gone undetected, and the discriminatory capacity was rather poor (area under the curve [AUC], 0.61-0.62). Specifically, 39% of the patients with rTOF and SCD compared with 24% of controls (P=.28) had a class IIa recommendation in the North American consensus document and 35% vs 27% (P=.37) in the European guidelines.9
Clinical predictors of malignant ventricular arrhythmias and/or sudden cardiac death in repaired tetralogy of Fallot
| Demographic factors | Male sex |
| Age | |
| Time since surgical repair | |
| Underlying anatomy | Anatomical complexity |
| Type of intervention | |
| Myocardial fibrosis | |
| Surgical repair | Age at the time of surgical repair |
| Previous palliative repair | |
| Right ventriculotomy incision | |
| Clinical history | Unexplained syncope |
| Fast palpitations | |
| Electrical monitoring | Sustained atrial arrhythmias |
| High-grade spontaneous ventricular extrasystoles | |
| Symptomatic nonsustained ventricular tachycardia | |
| Resting ECG | QRS duration>180 ms |
| QT dispersion>70 ms | |
| Chest X-ray | Cardiothoracic ratio >60% |
| Ergometry | Ventricular extrasystole on exercise |
| Hemodynamic factors | Moderate-severe RV systolic dysfunction |
| Severe dilatation of RV | |
| Disproportionate RV hypertrophy | |
| Moderate-severe LV systolic dysfunction | |
| Severe pulmonary regurgitation | |
| Residual obstruction of the right ventricular outflow tract | |
| Moderate-severe tricuspid regurgitation | |
| Increased LV end-diastolic pressure | |
| Electrophysiology study | Inducible sustained ventricular tachycardia |
| Arrhythmogenic isthmuses with slow conduction | |
| Prolonged RV activation time* | |
| HV interval> 55ms* |
HV, His-Purkinje interval; LV, left ventricle; RV, right ventricle.
In a recently published article in Revista Española de Cardiología, Rivas-Gándara et al.6 presented their work on electrophysiological predictors of ventricular arrhythmias in rTOF, in which they analyzed 56 consecutive patients who underwent electroanatomical mapping of the RV. The most common indication in the study (64%) was risk stratification prior to pulmonary valve replacement surgery; 11 of the 56 patients had had sustained VT before the procedure and another 10 patients had had inducible sustained VT on electrical stimulation. Multivariate analysis showed that the predictors independently associated with clinical or induced VT were: a) frequent ventricular extrasystoles (> 30/h) and/or nonsustained VT on resting ECG, 24-hour Holter, or interrogation of implanted defibrillator; b) RV activation time (in 10-ms increments), and c) prolonged HV interval (> 55 ms). The discriminatory capacity of the model was excellent (AUC=0.88).
The delayed RV electrical activation in patients with rTOF may be due to areas of block in the Purkinje system, surgical scars, or patches used for pulmonary stenosis repair, or structural damage (fibrosis) caused by hemodynamic changes, ventricular dilatation and/or hypertrophy, or ischemic damage. Jalal et al.10 recently demonstrated, using electroanatomical mapping and high-resolution magnetic resonance, that RV activation is prolonged in the whole ventricle, even in areas without scarring, and that the QRS duration only partially reflects the RV activation time. Prolongation of the HV interval is more intriguing. In patients with rTOF, the finding of right bundle branch block is very common, and some patients also have an anterior hemiblock. Prolongation of the HV interval indicates that there is also a delay in proximal activation, which affects the rapid conduction of the Purkinje network. Analogous to the abnormal LV contraction pattern observed in patients with left bundle branch block, patients with rTOF and right bundle branch block show asynchronous contraction of the RV. The dyssynchrony in turn leads to ventricular remodeling, which may worsen ventricular function even more and increase the risk of arrythmia. The results of this study, along with those published by Kapel et al.,11 support the assessment of other electrophysiological variables besides inducible sustained VT. The combination of electrophysiological variables, such as inducibility, prolonged conduction time and/or HV interval, and the presence of anatomical isthmuses with slow conduction might increase the predictive capacity of electrophysiological study.
The usefulness of the studies analyzing the role of electrophysiological study as a predictor of ventricular arrhythmias is limited by their retrospective design, small sample sizes and, most importantly, the low frequency of events, hampering meaningful estimation of predictive value. Performing a prospective study to analyze the predictive value of the electrophysiological variables discussed here, alone or in combination, would not be easy. The heterogeneity of the disease and the low annual rate of events would require large patient cohorts and considerably longer time periods to ensure adequate statistical power. Another important limitation is the inclusion of clinical episodes of sustained VT and sustained VT induced by programmed ventricular stimulation in the same category, factors that are not equivalent in terms of risk. Some studies have found a significant association between inducible VT and the incidence of arrhythmic events in patients with rTOF,12 while others (such as the study in reference) have not found a significant association between the two. The need to perform another invasive study to analyze these risk factors raises the question as to whether it can be avoided in certain patients with other noninvasive risk factors that have been shown to be independent predictors of inducible sustained VT on electrophysiological study (table 1). Recently, Ghonim et al.13 demonstrated that the 3-dimensional volume of late gadolinium enhancement on cardiac magnetic resonance also has good discriminatory capacity for predicting inducible ventricular arrythmias (AUC=0.81) and shows a good correlation with the extent of scarring on voltage mapping. This field, as well as the value of T1 mapping in predicting sustained VT (induced or spontaneous) and SCD, are currently under investigation.
However, the greatest limitation of this and other studies is the assumption that sustained VT can be used as an indirect marker of arrhythmic SCD. It is certainly true that many episodes of SCD are caused by VT, but, although the prevalence of VT in patients with rTOF is higher than in all the adult congenital heart diseases (reaching 15% of patients in some studies14), the annual incidence of arrhythmic SCD in this population is between 0.1% and 0.2%. The main causes of arrhythmic SCD in adults with congenital heart disease are Eisenmenger syndrome, unrepaired cyanotic heart disease, left heart lesions—principally aortic stenosis or coarctation of the aorta—congenital coronary artery anomalies, and severe systolic dysfunction of a systemic ventricle with right morphology, but the prevalence of clinical sustained VT in all these conditions is much lower than in rTOF.15 The potential mechanisms of ventricular arrhythmias are heterogeneous and highly variable depending on the underlying abnormality and the type—and timing—of surgical repair, and the consequences of sustained VT differ greatly depending on the arrhythmogenic mechanism. When the cause is related to diffuse myocardial fibrosis in ventricles with substantial structural damage, ventricular arrhythmias are poorly tolerated and often result in death. However, the likelihood of recovering from a macrore-entrant VT for young patients with preserved ventricular function is much higher. Recent data show that, in most patients with rTOF and serious ventricular arrhythmias, the conduction properties of the ventricular myocardium are normal and, although there are often functional lines of block, especially in the outflow tract, structural lines of block are rare.16 The current evidence indicates that in rTOF, most VT, whether spontaneous or induced, is caused by an arrhythmogenic anatomical isthmus (long, narrow, with reduced conduction velocity and low voltage), which facilitates preventive catheter or surgical ablation in the isthmic areas. However, prophylactic implantation of a cardioverter-defibrillator is still required in patients with rTOF and reduced left or right ventricular function and multiple risk factors, even if effective ablation is performed of the isthmuses with slow conduction that sustain VT.
In summary, serious ventricular arrhythmias, especially sustained VT, are a frequent cause of morbidity in adult patients with rTOF and can sometimes lead to SCD. Recent studies have improved our understanding of the pathophysiological mechanisms and the detection and prevention procedures for these serious complications. However, it is still unknown if implementing such procedures will result in a significant reduction in arrhythmic SCD in this young and vulnerable population.
FUNDINGNone.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to declare.