Atrial fibrillation constitutes a serious public health problem because it can lead to complications. Thus, the management of this arrhythmia must include not only its treatment, but antithrombotic therapy as well. The main goal is to determine the proportion of cases of undiagnosed atrial fibrillation and the proportion of patients not being treated with oral anticoagulants.
MethodsA multicenter, population-based, retrospective, cross-sectional, observational study. In all, 1043 participants over 60 years of age were randomly selected to undergo an electrocardiogram in a prearranged appointment. Demographic data, CHA2DS2-VASc and HAS-BLED scores, international normalized ratio results, and reasons for not receiving oral anticoagulant therapy were recorded.
ResultsThe overall prevalence of atrial fibrillation was 10.9% (95% confidence interval, 9.1%-12.8%), 20.1% of which had not been diagnosed previously. In the group with known atrial fibrillation, 23.5% of those with CHA2DS2-VASc≥2 were not receiving oral anticoagulant therapy, and 47.9% had a HAS-BLED score≥3. The odds ratio for not being treated with oral anticoagulation was 2.04 (95% confidence interval, 1.11-3.77) for women, 1.10 (95% confidence interval, 1.05-1.15) for more advanced age at diagnosis, and 8.61 (95% confidence interval 2.38-31.0) for a CHA2DS2-VASc score<2. Cognitive impairment (15.2%) was the main reason for not receiving oral anticoagulant therapy.
ConclusionsThe prevalence of previously undiagnosed atrial fibrillation in individuals over 60 years of age is 20.1%, and 23.5% of those who have been diagnosed receive no treatment with oral anticoagulants.
Keywords
Atrial fibrillation (AF) is a serious public health problem with a significant impact on health care costs.1 It is associated with severe complications2 such as stroke, systemic embolism, heart failure, and cognitive impairment, all of which leads to considerable morbidity and mortality2–4; thus, its early diagnosis and proper treatment are of great importance.5 We now know that stroke associated with AF implies greater severity, a higher mortality rate, and greater disability,6,7 which means a greater socioeconomic impact due to the costs derived from hospital admissions and home care required by the patients.8–10 Therefore, the management of patients with AF should include treatment not only of the fibrillation itself, but for the prevention of stroke and other thromboembolic events as well.11
Although oral anticoagulant therapy (OAT) has been effective in the primary and secondary prevention of embolisms in AF patients with valvular heart disease since 1947,12 their use in patients with nonvalvular AF was not recommended13 until after 1986, whereas, at present14–16 it is recommended as the optimal choice. The introduction of new tools, such as the CHADS2 (congestive heart failure, hypertension, age≥75 [doubled], diabetes, stroke [doubled]) risk score,17 its upgrade to CHA2DS2-VASc (congestive heart failure, hypertension, age≥75 [doubled], diabetes, stroke [doubled]-vascular disease and sex category [female]),18-20 to stratify patients according to their risk, and the HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly [>65 years], drugs/alcohol concomitantly) score to evaluate the risk of bleeding,21–23 enables more efficient clinical decision-making with regard to both the indications for OAT and the type of therapy. Even so, today it is common to find a significant proportion of patients with AF who do not receive OAT.24
ObjectivesThe main objectives of this study are to estimate the prevalence of undiagnosed AF among individuals over the age of 60 years in the Baix Ebre region, located in the province of Tarragona, in northeastern Spain, and the percentage of patients with AF who do not receive OAT. The secondary objectives are to establish the distribution of the population with AF according to the CHA2DS2-VASc and HAS-BLED scores, identify the characteristics of the patients with AF not receiving OAT, and to determine the reasons for the failure to administer OAT to patients in whom it is indicated.
METHODSStudy DesignThis report deals with a multicenter, population-based, retrospective, cross-sectional, observational study. Paroxysmal AF is understood to be fibrillation with a duration of less than 7 days; persistent AF is that having a duration of more than 7 days but less than 12 months; and permanent AF is that which persists indefinitely.25 Our study includes AF in any of its presentations as documented in the medical records. AF was considered to be undiagnosed when the electrocardiographic recording of an individual was positive, but AF was not reported in the medical record. For the first of the main objectives, we obtained a randomized sample of 1043 patients over the age of 60 years who resided in the Baix Ebre region, with a 95% confidence level and a margin of error of 5%. From each primary care center or medical office, we selected a number of cases proportional to the patients over 60 years of age assigned to that site (Table 1). The selected patients were contacted by telephone and asked to provide signed informed consent to participate and undergo an electrocardiogram (ECG) in their primary care center. During the appointment, the patient's medical record was reviewed, after which the investigators and the cardiologist performed and interpreted the ECG.
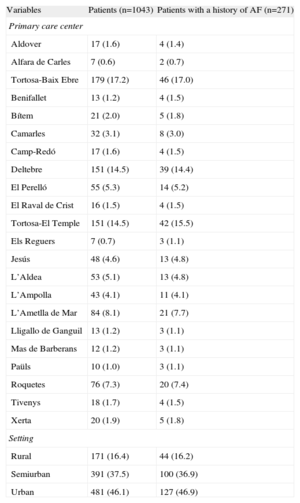
Descriptive Analysis of Patient Distribution According to Locality and Setting
| Variables | Patients (n=1043) | Patients with a history of AF (n=271) |
| Primary care center | ||
| Aldover | 17 (1.6) | 4 (1.4) |
| Alfara de Carles | 7 (0.6) | 2 (0.7) |
| Tortosa-Baix Ebre | 179 (17.2) | 46 (17.0) |
| Benifallet | 13 (1.2) | 4 (1.5) |
| Bítem | 21 (2.0) | 5 (1.8) |
| Camarles | 32 (3.1) | 8 (3.0) |
| Camp-Redó | 17 (1.6) | 4 (1.5) |
| Deltebre | 151 (14.5) | 39 (14.4) |
| El Perelló | 55 (5.3) | 14 (5.2) |
| El Raval de Crist | 16 (1.5) | 4 (1.5) |
| Tortosa-El Temple | 151 (14.5) | 42 (15.5) |
| Els Reguers | 7 (0.7) | 3 (1.1) |
| Jesús | 48 (4.6) | 13 (4.8) |
| L’Aldea | 53 (5.1) | 13 (4.8) |
| L’Ampolla | 43 (4.1) | 11 (4.1) |
| L’Ametlla de Mar | 84 (8.1) | 21 (7.7) |
| Lligallo de Ganguil | 13 (1.2) | 3 (1.1) |
| Mas de Barberans | 12 (1.2) | 3 (1.1) |
| Paüls | 10 (1.0) | 3 (1.1) |
| Roquetes | 76 (7.3) | 20 (7.4) |
| Tivenys | 18 (1.7) | 4 (1.5) |
| Xerta | 20 (1.9) | 5 (1.8) |
| Setting | ||
| Rural | 171 (16.4) | 44 (16.2) |
| Semiurban | 391 (37.5) | 100 (36.9) |
| Urban | 481 (46.1) | 127 (46.9) |
AF, atrial fibrillation.
The data are expressed as no. (%).
For the second of the main objectives, we obtained a second randomized sample (n=271) consisting of patients with known AF, as documented in their medical records. This sample included the 64 patients with diagnosed and confirmed AF of the first sample. From each primary care center or medical office, we selected, from the group of patients with previously known AF, a number of cases proportional to the individuals over 60 years of age assigned to that site. The data corresponding to the study variables were collected using a Microsoft Access electronic form.
Study VariablesThe patients were distinguished by their patient identification code (center number and patient number for this study).
With regard to sociodemographic and clinical variables, patients living in towns of fewer than 1000 inhabitants were considered to reside in a rural setting; municipalities of between 1000 and 10 000 inhabitants were considered to be semiurban, and those of over 10 000 inhabitants, urban.
The following data were collected in relation to the CHA2DS2-VASc score: date of birth, sex, diagnosis of hypertension, diagnosis of diabetes mellitus, history of heart failure, previous stroke, previous transient ischemic attack, previous thromboembolism and history of vascular disease (previous myocardial infarction, complex aortic plaque, peripheral arterial disease, including previous revascularization, amputation due to peripheral arterial disease, or angiographic evidence of peripheral arterial disease).
Additional variables were collected in relation to the HAS-BLED score: most recent arterial blood pressure (mmHg); abnormal renal function (chronic dialysis, renal transplant, or serum creatinine level of 200mol/L or higher); abnormal liver function (chronic liver disease—such as cirrhosis—or significant biochemical evidence of liver disease—eg, bilirubin 2-fold higher than the normal limit, in association with AMT/AAT/APT 3-fold higher than normal); history of or predisposition to bleeding (previous history of bleeding and/or predisposition to bleeding); labile international normalized ratio (INR) (unstable/elevated INR or less than 60% of the time within therapeutic range); and concomitant chronic drug use (antiplatelet agents, nonsteroidal anti-inflammatory drugs, and alcohol abuse).
We recorded the treatment being received on the day the medical record was analyzed (categories: no treatment, antiplatelet agent, oral anticoagulant, and parenteral anticoagulant).
The reason for not prescribing or for discontinuing OAT was included. The categories were the impossibility of undergoing periodic monitoring, risk of hemorrhage, patient refusal or preference, cognitive impairment, frequent falls, poorly controlled hypertension, multidrug therapy, not appropriate according to the CHA2DS2-VASc score, not indicated, and others. To meet the objective, 28 randomly selected primary care professionals were interviewed and the medical records included in addressing the second objective were reviewed. A questionnaire especially designed for this study was distributed via e-mail to each of the randomly selected professionals (n=30). They were given 30 days to respond to the questionnaire. When no response was received, we requested the collaboration of the physician selected as a substitute. The same investigators reviewed the medical records. Electronic data collection was carried out using a specifically designed Microsoft Access form.
For each of the INR values of the preceding 3 months, we recorded the date as DD/MM/YYYY format and the percentage of time in which the values were within therapeutic range (2-3).
Statistical AnalysisA descriptive analysis of the variables was carried out using minimum, mean, standard deviation, and maximum to express the continuous variables and the number of cases and percentage of patients to express the categorical variables, with their confidence intervals, considering a finite reference population. Risk factor distribution was compared in the groups of individuals classified on the basis of their diagnosis of AF. The results were obtained using the chi-squared test or Fisher exact test for categorical values, or analysis of variance or the Kruskal-Wallis test for continuous variables. Logistic regression analysis was performed with the 271 patients of the second sample to analyze possible factors that characterize the population diagnosed as having AF but not receiving OAT. The discrimination capacity was calculated by means of the ROC (receiver operating characteristic curve and the calibration properties using the Hosmer-Lemeshow test, with the 95% confidence interval (95%CI) for the coefficients of the model. The dichotomous dependent variable was the condition of receiving TAO or not.
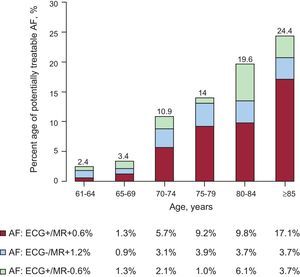
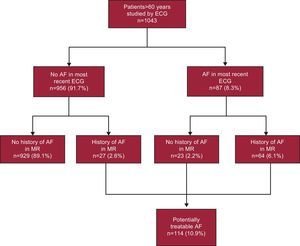
RESULTSFigure 1 shows the prevalence of AF stratified according to age groups. The mean age of the study population (n=1043) was 78.9 years (7.3 years). The prevalence of AF was 10.9% (95%CI, 9.1%-12.8%). It increased with age, from 2.4% among patients aged 61 years to 64 years to 24.4% among patients aged 85 years and older. At this age, the trend is inverted in favor of women and the maximum prevalence, 26.2%, is reached. Figure 2 illustrates the different possible situations, showing that 23 (20.1%) of 114 confirmed or recorded cases of AF would have been previously undiagnosed and that approximately 1 in 45 individuals (23 of 1043 participants) would have undiagnosed AF.
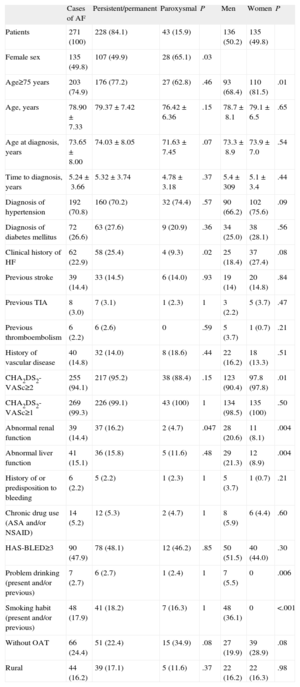
Table 2 lists the descriptive variables and those related to the CHA2DS2-VASc and HAS-BLED scores, as well as the history of vascular disease, the treatment, and the residential setting of the sample population with known AF. The distribution of the CHA2DS2-VASc score was centered around 4 points, and 94.1% of the participants had a score of 2 or more. With respect to the HAS-BLED score, 14.4% and 15.1% of the participants had abnormal renal function and abnormal liver function, respectively. In all, 47.9% had HAS-BLED scores of 3 or higher. The CHA2DS2-VASc score was positively correlated with the HAS-BLED score (Spearman's rho=0.33). The incidence of hypertension and of diabetes in the study population were 70.8% and 26.6%, respectively; 14.8% of the participants had some type of documented vascular disease and nearly 90% had previously had a myocardial infarction. Taking the risk factors as a whole, the great majority of the population studied had 3 risk factors or fewer.
Descriptive Analysis of the Demographic Variables and Those Related to the CHA2DS2-VASc and HAS-BLED Scores and Other Risk Factors for Atrial Fibrillation
| Cases of AF | Persistent/permanent | Paroxysmal | P | Men | Women | P | |
| Patients | 271 (100) | 228 (84.1) | 43 (15.9) | 136 (50.2) | 135 (49.8) | ||
| Female sex | 135 (49.8) | 107 (49.9) | 28 (65.1) | .03 | |||
| Age≥75 years | 203 (74.9) | 176 (77.2) | 27 (62.8) | .46 | 93 (68.4) | 110 (81.5) | .01 |
| Age, years | 78.90±7.33 | 79.37±7.42 | 76.42±6.36 | .15 | 78.7±8.1 | 79.1±6.5 | .65 |
| Age at diagnosis, years | 73.65±8.00 | 74.03±8.05 | 71.63±7.45 | .07 | 73.3±8.9 | 73.9±7.0 | .54 |
| Time to diagnosis, years | 5.24±3.66 | 5.32±3.74 | 4.78±3.18 | .37 | 5.4±309 | 5.1±3.4 | .44 |
| Diagnosis of hypertension | 192 (70.8) | 160 (70.2) | 32 (74.4) | .57 | 90 (66.2) | 102 (75.6) | .09 |
| Diagnosis of diabetes mellitus | 72 (26.6) | 63 (27.6) | 9 (20.9) | .36 | 34 (25.0) | 38 (28.1) | .56 |
| Clinical history of HF | 62 (22.9) | 58 (25.4) | 4 (9.3) | .02 | 25 (18.4) | 37 (27.4) | .08 |
| Previous stroke | 39 (14.4) | 33 (14.5) | 6 (14.0) | .93 | 19 (14) | 20 (14.8) | .84 |
| Previous TIA | 8 (3.0) | 7 (3.1) | 1 (2.3) | 1 | 3 (2.2) | 5 (3.7) | .47 |
| Previous thromboembolism | 6 (2.2) | 6 (2.6) | 0 | .59 | 5 (3.7) | 1 (0.7) | .21 |
| History of vascular disease | 40 (14.8) | 32 (14.0) | 8 (18.6) | .44 | 22 (16.2) | 18 (13.3) | .51 |
| CHA2DS2-VASc≥2 | 255 (94.1) | 217 (95.2) | 38 (88.4) | .15 | 123 (90.4) | 97.8 (97.8) | .01 |
| CHA2DS2-VASc≥1 | 269 (99.3) | 226 (99.1) | 43 (100) | 1 | 134 (98.5) | 135 (100) | .50 |
| Abnormal renal function | 39 (14.4) | 37 (16.2) | 2 (4.7) | .047 | 28 (20.6) | 11 (8.1) | .004 |
| Abnormal liver function | 41 (15.1) | 36 (15.8) | 5 (11.6) | .48 | 29 (21.3) | 12 (8.9) | .004 |
| History of or predisposition to bleeding | 6 (2.2) | 5 (2.2) | 1 (2.3) | 1 | 5 (3.7) | 1 (0.7) | .21 |
| Chronic drug use (ASA and/or NSAID) | 14 (5.2) | 12 (5.3) | 2 (4.7) | 1 | 8 (5.9) | 6 (4.4) | .60 |
| HAS-BLED≥3 | 90 (47.9) | 78 (48.1) | 12 (46.2) | .85 | 50 (51.5) | 40 (44.0) | .30 |
| Problem drinking (present and/or previous) | 7 (2.7) | 6 (2.7) | 1 (2.4) | 1 | 7 (5.5) | 0 | .006 |
| Smoking habit (present and/or previous) | 48 (17.9) | 41 (18.2) | 7 (16.3) | 1 | 48 (36.1) | 0 | <.001 |
| Without OAT | 66 (24.4) | 51 (22.4) | 15 (34.9) | .08 | 27 (19.9) | 39 (28.9) | .08 |
| Rural | 44 (16.2) | 39 (17.1) | 5 (11.6) | .37 | 22 (16.2) | 22 (16.3) | .98 |
AF, atrial fibrillation; ASA, acetylsalicylic acid; HF, heart failure; NSAID, nonsteroidal anti-inflammatory drugs; OAT, oral anticoagulation therapy; TIA, transient ischemic attack.
The data are expressed as mean±standard deviation or no. (%).
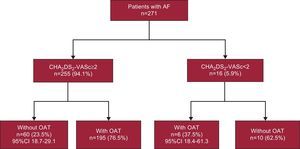
As can be seen in Figure 3, the subgroup of untreated participants consisted of the 23.5% in whom OAT was indicated because their CHA2DS2-VASc score was 2 or more and the 37.5% in which it was not indicated because their CHA2DS2-VASc score was less than 2. Higher scores, male sex, and age were associated with OAT. Of the patients with a history of AF that was not confirmed by the most recent electrocardiogram, 37.5% received OAT (95%CI, 21.1%-57.3%). None of the patients with undiagnosed AF were being treated with OAT.
In all, 86.7% of the participants receiving OAT were monitored in referral primary care centers that use a software program to indicate dose adjustments. Of the 895 INR values collected, 38.0% were outside the therapeutic range; there were no significant differences (P=.47) between primary care centers and referral hospitals. In the 195 cases with available measurements, the mean was 2.60 (0.44). While 17.9% of the participants had a mean value outside the therapeutic range, 80.5% had presented with at least one INR value outside this range (2-3) during the preceding 3 months (Table 3). The mean percentage of the preceding 3 months during which the patients had INR values within the therapeutic range was 69.1% (27.9%). In all, 32.3% of the patients had INR values within the therapeutic range less than 60% of the time.
Descriptive Analysis of International Normalized Ratio<2 and >3 in Patients Diagnosed as Having Atrial Fibrillation
| Variables | Cases, n (%) |
| Patients with INR values outside the therapeutic range | |
| No | 38 (19.5) |
| Yes | 157 (80.5) |
| INR values outside the therapeutic range | |
| No | 555 (62.0) |
| Yes | 340 (38.0) |
INR, international normalized ratio.
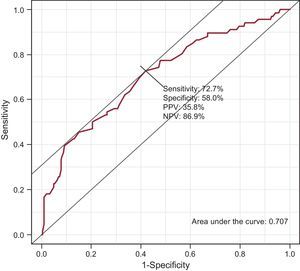
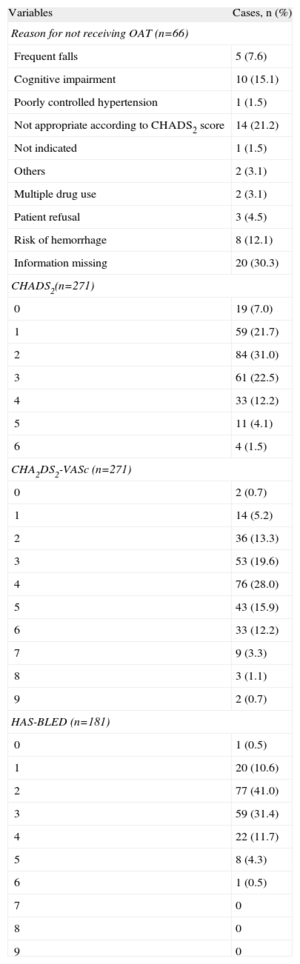
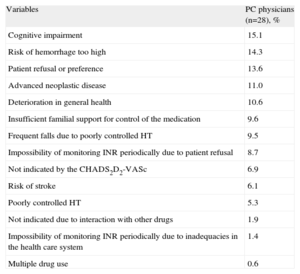
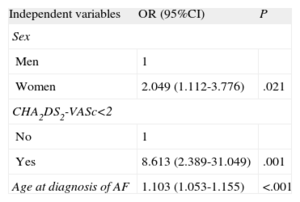
Among the patients in whom OAT was recommended (Table 4) on the basis of the review of their medical records, “not appropriate according to the CHA2DS2-VASc score”, “cognitive impairment”, and “risk of hemorrhage” were the most frequent reasons for not prescribing it. We should point out that it was not possible to obtain information in 30.3% of the cases. According to the responses of the primary care professionals (Table 5), the most common reasons for not administering or for discontinuing OAT were cognitive impairment (15.1%), a too-high risk of hemorrhage (14.3%), and patient refusal or preference (13.6%). Failure to receive OAT was associated with being female (odds ratio [OR]=1.82; 95%CI, 1.01-3.30), a diagnosis of hypertension (OR=0.50; 95%CI, 0.27-0.93), age at the time AF was diagnosed (OR=1.07; 95%CI, 1.03-1.11), and CHA2DS2-VASc score of <2. The risk of not receiving OAT was higher among women and increased with the age at which AF was diagnosed (Table 6). All the coefficients of the model were significant (P<.001), explaining 13.9% of the variability. The discrimination properties according to the area under the curve (0.707) and calibration properties (chi-squared=6.9; P=.54) show that the model fit well with the data (Fig. 4).
Descriptive Analysis of the Treatment and Indicators of Stroke Risk in Patients Diagnosed as Having Atrial Fibrillation (n=271)
| Variables | Cases, n (%) |
| Reason for not receiving OAT (n=66) | |
| Frequent falls | 5 (7.6) |
| Cognitive impairment | 10 (15.1) |
| Poorly controlled hypertension | 1 (1.5) |
| Not appropriate according to CHADS2 score | 14 (21.2) |
| Not indicated | 1 (1.5) |
| Others | 2 (3.1) |
| Multiple drug use | 2 (3.1) |
| Patient refusal | 3 (4.5) |
| Risk of hemorrhage | 8 (12.1) |
| Information missing | 20 (30.3) |
| CHADS2(n=271) | |
| 0 | 19 (7.0) |
| 1 | 59 (21.7) |
| 2 | 84 (31.0) |
| 3 | 61 (22.5) |
| 4 | 33 (12.2) |
| 5 | 11 (4.1) |
| 6 | 4 (1.5) |
| CHA2DS2-VASc (n=271) | |
| 0 | 2 (0.7) |
| 1 | 14 (5.2) |
| 2 | 36 (13.3) |
| 3 | 53 (19.6) |
| 4 | 76 (28.0) |
| 5 | 43 (15.9) |
| 6 | 33 (12.2) |
| 7 | 9 (3.3) |
| 8 | 3 (1.1) |
| 9 | 2 (0.7) |
| HAS-BLED (n=181) | |
| 0 | 1 (0.5) |
| 1 | 20 (10.6) |
| 2 | 77 (41.0) |
| 3 | 59 (31.4) |
| 4 | 22 (11.7) |
| 5 | 8 (4.3) |
| 6 | 1 (0.5) |
| 7 | 0 |
| 8 | 0 |
| 9 | 0 |
OAT, oral anticoagulation therapy.
Reasons for Not Prescribing or for Discontinuing Oral Anticoagulation Therapy in Patients With Atrial Fibrillation
| Variables | PC physicians (n=28), % |
| Cognitive impairment | 15.1 |
| Risk of hemorrhage too high | 14.3 |
| Patient refusal or preference | 13.6 |
| Advanced neoplastic disease | 11.0 |
| Deterioration in general health | 10.6 |
| Insufficient familial support for control of the medication | 9.6 |
| Frequent falls due to poorly controlled HT | 9.5 |
| Impossibility of monitoring INR periodically due to patient refusal | 8.7 |
| Not indicated by the CHADS2D2-VASc | 6.9 |
| Risk of stroke | 6.1 |
| Poorly controlled HT | 5.3 |
| Not indicated due to interaction with other drugs | 1.9 |
| Impossibility of monitoring INR periodically due to inadequacies in the health care system | 1.4 |
| Multiple drug use | 0.6 |
HT, hypertension; INR, international normalized ratio; PC, primary care.
Logistic Regression Analysis With Factors That Characterize the Population Not Treated With Anticoagulants Among the Patients Diagnosed as Having Atrial Fibrillation and Having a CHA2DS2-VASc Score of 2 or More. Variables Significant in the Multivariate Model (n=60)
| Independent variables | OR (95%CI) | P |
| Sex | ||
| Men | 1 | |
| Women | 2.049 (1.112-3.776) | .021 |
| CHA2DS2-VASc<2 | ||
| No | 1 | |
| Yes | 8.613 (2.389-31.049) | .001 |
| Age at diagnosis of AF | 1.103 (1.053-1.155) | <.001 |
95%CI, 95% confidence interval; AF, atrial fibrillation; OR, odds ratio.
The prevalence of AF in our outpatient population is similar to that reported in other studies,3,24,26,27 with an absolute prevalence of undiagnosed AF of 2.2%,equivalent to 20.1% of the overall prevalence of AF. This is higher than in the reports of other studies, which range from 0.49% to 1.7%24,28–30 when diagnosed by means of standard ECG, but lower than the AF incidence (30%) detected by continuous monitoring in patients with risk factors for stroke.31 The prevalence of AF in the community is probably underestimated, as a consequence of the failure to detect and diagnose it. Although there are studies6,27 on the prevalence of AF among stroke patients that appear to confirm a possible underdiagnosis of the condition, they cannot be compared with the findings in the general population. While the data confirm the evident age-related increase in the prevalence of persistent AF and demonstrate that hypertension is the most frequently associated cardiovascular risk factor,3,32,33 together with the presence of cardiovascular disease,34 there is not sufficient evidence regarding the procedures that may be most effective35 for achieving an early diagnosis of AF and reducing the associated risks. The proposals range from opportunistic screening36,37 to the monitoring of patients who have no history of AF, but have 1 or more points on the CHA2DS2-VASc score.38,39
Together, undiagnosed and untreated AF constitute 43.9% of the overall prevalence, a finding that defines the magnitude of the problem, which differs from that of known but untreated AF. Our findings are along the lines of the results reported in the Plan Director para la atención de la enfermedad cerebrovascular en Cataluña (Executive Plan for Cerebrovascular Disease in Catalonia [Spain])40—the study shows a higher percentage of patients treated with OAT than that indicated in the published evidence24,27,41–43 and the percentage of time in which the INR values of the patient are within therapeutic range is similar or superior to those reported elsewhere,44 depending on the center and the country (for example, in the United Kingdom and in Sweden, the participating centers reached percentages of time with the patient within therapeutic range of 72% and 77%, respectively). However, we would still be far from obtaining results of nearly 80% of our patients, in accordance with the principle of applicability of clinical practice guidelines in our routine clinical practice.45–47 Thus, we should study in depth the reasons for OAT administration in approximately one third of the patients in whom it would not be indicated by the CHA2DS2-VASc score, since it may constitute an avoidable risk.
Given the evidence of the increase in the absolute benefit of OAT as patients age, together with the availability of scoring systems that enable us to stratify the risk and that facilitate decision making with regard to antithrombotic prophylaxis, primary care physicians play an essential role in the screening, early detection, risk assessment, and prescription and adjustment of OAT in patients with AF. Thus, it would be interesting to establish the reasons for which a patient for whom OAT is indicated does not receive it. We cannot rule out a subjectivity bias involving “cognitive impairment” and “risk of hemorrhage.” Given that the new scoring systems lead to a tendency to include patients, we consider it appropriate that a debate about maintaining OAT should go beyond strictly clinical criteria and consider the benefits and opportunities generated by the new oral anticoagulants from the societal perspective.48 Finally, the systematic use of the HAS-BLED score16,20,49 could reduce the risks of hemorrhage, while maintaining the benefits of OAT. The median HAS-BLED score in our population and the time within therapeutic range are similar to those reported elsewhere.20 It is worthy of note that the coincidence in the majority of the factors used in the two scores can be highly predictive not only of the risk of hemorrhage, but of the incidence of cardiovascular events in AF patients receiving OAT as well, as has been demonstrated by other authors.49
Compared with the regression analysis results, the risk of not receiving OAT is higher among women and increases with age, but not with the time elapsed since the diagnosis of AF, which coincides with earlier findings.50 On the other hand, while there are reports of differences in the management of AF in men and women51 that could be especially justified by the more advanced age of the latter—although not exclusively because of their clinical characteristics—the use of scoring systems to stratify the risk of bleeding and cardiovascular risk should improve the standard prescription of OAT and reduce the effect of the progressive aging of the population.
CONCLUSIONSThe aging of the population is associated with a progressive increase in the prevalence of AF (24.4%), and it is estimated that approximately 20% of the cases have not been diagnosed.
In all, 23.5% of the patients with AF do not receive OAT; to this group we should add part of the population not receiving OAT but having undiagnosed AF.
The risk of not receiving OAT increases with age at the time of diagnosis of AF and is higher among women.
CONFLICTS OF INTERESTThe present project was sponsored by the Pla Director de la Malaltia Vascular Cerebral of the Departament de Salut of the Generalitat de Catalunya, Spain, and the aim was to determine the true status of AF and its management in the health care setting in the geographic region of Catalonia. This study was approved by the Spanish Medicines and Health Products Agency (AEMPS, Clinical Trial Registration no. MGL-ANT-2011-01) and the Clinical Research Ethics Committee (CEIC) of the IDIAP (Instituto de Investigación en Atención Primaria) Jordi Gol (5011/011), and was financed by Boehringer Ingelheim España, S.A.