To compare the pharmacodynamics of the CNIC polypill (atorvastatin 40mg/ramipril 10mg/aspirin 100mg) in terms of low-density lipoprotein cholesterol (LDL-C) and systolic blood pressure (SBP), with the corresponding reference products (atorvastatin and ramipril).
MethodsThis was a multicenter, randomized, open-label, and parallel 3-arm study comparing the effect of the CNIC polypill vs ramipril 10mg and atorvastatin 40mg on SBP and LDL-C. The coprimary endpoints were differences in the adjusted mean 24-hour SBP (using ambulatory BP measurement) and LDL-C during the study period estimated using an ANCOVA model.
ResultsOf the 241 patients included in the per protocol population, 84 received the CNIC polypill (group A), 84 atorvastatin (group B), and 73 ramipril (group C). SBP decreased from 139.3±12.5 to 133.2±12.9mmHg in group A and from 138.1±11.9 to 134.0±12.8mmHg in group C (baseline adjusted mean difference for the decrease in SBP was 1.77mmHg (90%CI, −0.5 to 4.0) in favor of group A, without reaching statistical significance. LDL-C was reduced by 33.9±21.6 and 29.2±25.8mg/dL in groups A and B, respectively (baseline adjusted mean difference for the decrease in LDL-C was 7.0% (90%CI, 1.5–12.4), a significantly greater decrease with the polypill). The 3 treatments were well tolerated.
ConclusionsThe results of this study rule out a negative effect on blood pressure of the interaction between the components of the CNIC polypill. The reduction in LDL-C was greater in the CNIC polypill group, suggesting a synergistic effect of the components.
Keywords
Cardiovascular disease is the main cause of premature death worldwide in adults, accounting for more than 30% of all deaths.1 In the last few decades, reduced cardiovascular mortality has been reported in developed countries, thus highlighting the importance of secondary prevention.2 International guidelines indicate that the most effective cardioprotective drug therapy for secondary prevention includes angiotensin-converting enzyme inhibitors (ACEi), statins, and low-dose aspirin.3–5 Unfortunately, only around 50% of patients with established cardiovascular disease from high-income countries and up to 5% from low-income countries are receiving long-term therapy with guideline-recommended blood pressure (BP)–lowering drugs, statins, and antiplatelets.6 In addition, the EUROASPIRE V registry has recently shown that only 48% and 29% of patients with coronary disease achieve BP and low-density lipoprotein cholesterol (LDL-C) targets, respectively.7 Therefore, it is mandatory to improve the achievement of goals for cardiovascular risk factors.8,9
Various studies have shown that combining statins, antihypertensive agents, and aspirin into 1 pill facilitates the achievement of BP, LDL-C, and antiplatelet therapy targets simultaneously, when compared with treatment based on 1 or 2 of these drugs.10–14 Therefore, combining these 3 drugs in the form of a polypill could be a key scalable strategy for containing the atherosclerotic cardiovascular disease pandemic.8,9 In fact, guidelines suggest that the use of the polypill may be considered to enhance adherence and improve risk factor control.3–5,15,16
The study of polypills in various clinical trials (13 to date, including nearly 10000 patients) has revealed improved control of risk factors.10,12,13 The Centro Nacional de Investigaciones Cardiovasculares (CNIC) polypill contains ramipril, atorvastatin, and low-dose aspirin,17 and while bioequivalence between the polypill and the 3 drugs given alone has been demonstrated,17 no studies have been published that assess possible interactions between the 3 components when administered together in a polypill. The current study aimed to assess pharmacodynamic interactions between the components of the CNIC polypill in terms of reduction of LDL-C and systolic BP (SBP) compared with monotherapy based on the reference products.
METHODSThis was a multicenter, randomized, open-label, repeated-dose, parallel enriched 3-arm study. The main objective was to compare pharmacodynamic interactions in the CNIC polypill (effect on SBP and LDL-C) and the reference products, namely, atorvastatin and ramipril. Secondary objectives were to compare diastolic BP (DBP), high-density lipoprotein (HDL) cholesterol (HDL-C), total cholesterol, and triglycerides between the groups at the end of the study. Finally, the safety and tolerability of the drugs were also evaluated. Patients were recruited from 41 different centers/hospitals in the United States.
The main inclusion criteria were as follows: male or female participants aged ≥ 18 and <75 years; stage 1 hypertension (SBP/DBP: 140-159/90-99 mmHg) or stage 2 hypertension (SBP/DBP: 160-179/100-109 mmHg); and LDL-C ≥ 100 mg/dL. The main exclusion criteria were as follows: body mass index> 35 kg/m2; SBP <140mmHg and/or DBP <90 mmHg; severe hypertension (SBP> 180mmHg and DBP> 110 mmHg); LDL-C <100 mg/dL; triglycerides ≥ 400 mg/dL; and a history of previous cardiovascular events. The study was conducted according to the ethical principles of the Declaration of Helsinki as adopted by the World Medical Assembly in 1964 (and subsequent revisions) and was approved by the ethics committee and the Food and Drug Administration prior to initiation. All participants provided their written informed consent following a detailed explanation of the objectives and the protocol.
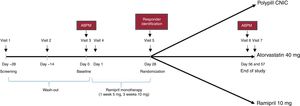
The design of the study is presented in figure 1. The study was set in 3 consecutive phases. Phase 1 consisted of a 4-week wash-out period followed by a 4-week run-in period based on monotherapy with ramipril (5mg ramipril for 1 week and 10mg ramipril for 3 weeks) to identify responders (phase 2). A responder was defined as a patient with a decrease in SBP ≥ 15mmHg after the run-in with ramipril (office measurement). Only those patients who the investigator deemed safe to enter into the wash-out period were included.
Patients who responded to ramipril entered phase 3 of the study and were then randomized 1:1:1 to 1 of the 3 treatment arms for 4 weeks; CNIC polypill (aspirin 100mg, atorvastatin 40mg, and ramipril 10 mg), Altace (ramipril 10 mg), or Lipitor (atorvastatin 40mg). During the study, BP was monitored and if a patient had a SBP> 180mmHg or a DBP> 110mmHg (confirmed in 2 measurements), the patient was withdrawn from the study according to the investigator's criteria.
Office BP measurement was performed at the end of the ramipril monotherapy and identification of responders (visit 5, day 28±2 days). This measurement consisted of an average of 2 BP readings taken 2minutes after the patient had sat for 5minutes in a quiet environment. SBP for determining the coprimary endpoint was assessed by means of 24-hour ambulatory BP measurement at baseline (visit 3) and at the end of study (visit 7). Patients had to attend the study site 24hours after each of these visits for device removal. If for technical reasons the readings failed, an additional 24-hour ambulatory BP reading was done within 24 to 72hours of the scheduled visit. The 24-hour ambulatory BP reading was performed by a central laboratory. The procedures for the correct management of the 24-hour ambulatory BP were detailed in a specific guide.
Changes in LDL-C were calculated using the Friedewald formula between baseline (visit 5) and the end of study (visit 7).
The investigator closely monitored any adverse events and adopted the necessary clinical measures to ensure the safety of the study participants. All adverse events, serious adverse events, and deaths related to the investigational medicinal product were recorded, as were adverse events leading to premature discontinuation from the study.
Statistical analysisAssuming a minimum decrease in SBP of 15mmHg and a mean decrease of 17.5mmHg with a standard deviation±standard deviation of 9mmHg and a true ratio of T/R=1 (where T is treatment with the cardiovascular fixed dose combination pill and R is treatment with ramipril 10 mg), approximately 80 participants per arm were needed to show that the 90% confidence interval (90%CI) was within the equivalence margins of 80% to 125%. This number of participants was sufficient to demonstrate PD equivalence (decrease in LDL-C) of the cardiovascular fixed-dose combination pill and atorvastatin 40mg, the absolute equivalence margins being±6%.
Categorical data are presented as absolute and relative frequencies, and continuous variables as mean, median, standard deviation, minimum, maximum, and number of patients with an observation (n). The inferential analysis was limited to blood pressure (SBP and DBP, ambulatory BP measurement) and lipid profile variables (LDL-C, very LDL-C, HDL-C, total cholesterol, and triglycerides). Other variables were analyzed descriptively. All statistical tests were 2-sided and performed using a 5% significance level. For the BP and lipid profile variables, estimates for treatment effects and the corresponding 90%CI were provided.
The coprimary pharmacodynamic endpoints of this study were differences in the baseline adjusted mean 24-hour SBP results using ANCOVA model (using ambulatory BP measurement) between baseline (visit 3) and the final visit (visit 7) of the treatment period and the difference in LDL-C levels between baseline (visit 5) and the final visit (visit 7) of the treatment period.
Between-group differences in incremental SBP and LDL-C values after treatment were assessed using analysis of covariance, including fixed terms for treatment, center, and baseline SBP or LDL-C accordingly as covariates.
Pharmacodynamic equivalence between the CNIC polypill and the reference medication was to be declared if the 90%CI for the ratio obtained for difference in the mean changes in the 24-hour SBP fell within an acceptance interval of 0.8 to 1.25.
For LDL-C, the equivalence margin for the absolute difference in relative changes between the baseline and final visits was±6%.
The secondary endpoints of this study were the difference in the adjusted mean 24-hour DBP results (using ambulatory BP measurement) between baseline (visit 3) and the final visit (visit 7) of the treatment period; and the difference in HDL-C, total cholesterol, and triglyceride levels between baseline (visit 5) and the final visit (visit 7) of the treatment period. All the analyses were carried out using Statistical Analysis Software v9.2.
The per protocol population included all randomized participants who were adherent to the study medication (80%-120%), had at least baseline and postbaseline coprimary endpoint measurements, both for SBP and LDL-C, and who had no major protocol deviations. The per protocol population was used for the main analysis. The safety population included all participants who received at least 1 dose of the investigational medicinal product. The modified intention-to-treat population included all randomized patients who received at least 1 dose of the investigational medicinal product and underwent a postbaseline primary endpoint measurement. The modified intention-to-treat population was used for the sensitive analysis to rule out the impact of adherence on the results.
Summaries and analyses of primary and secondary pharmacodynamic endpoints were conducted for the per protocol and the modified intention-to-treat population. Safety and tolerability data are presented for the safety population.
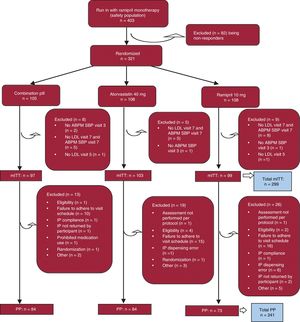
RESULTSThe study flow chart is shown in figure 2. A total of 403 participants were enrolled. Of these, 321 were randomized: 105 participants to the CNIC polypill arm and 108 participants each to the atorvastatin 40mg and ramipril 10mg arms were included in the safety analysis. The per protocol population included 241 participants with no major protocol deviations and adherence to treatment (80%-120%), with 84 participants in the CNIC polypill arm, 84 participants in the atorvastatin arm, and 73 participants in the ramipril arm. No patient was withdrawn from the study due to severe hypertension.
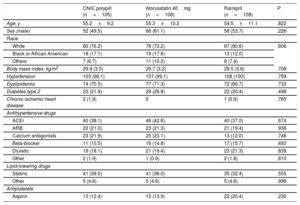
The demographic characteristics and prior treatments in the safety population are summarized in table 1. The groups were well balanced. Responders to ramipril in the run-in phase showed a mean±standard deviation decrease in SBP of 16.2±1.4mmHg (CNIC polypill group), 19.6±12.9mmHg (atorvastatin group), and 19.2±12.8mmHg (ramipril group).
Baseline clinical characteristics of the study population (safety population)
| CNIC polypill (n=105) | Atorvastatin 40mg (n=108) | Ramipril (n=108) | P | |
|---|---|---|---|---|
| Age, y | 55.2±9.2 | 55.3±10.2 | 54.5±11.1 | .822 |
| Sex (male) | 52 (49.5) | 66 (61.1) | 58 (53.7) | .226 |
| Race | ||||
| White | 80 (76.2) | 78 (72.2) | 87 (80.6) | .606 |
| Black or African American | 18 (17.1) | 19 (17.6) | 13 (12.0) | |
| Others | 7 (6.7) | 11 (10.2) | 8 (7.4) | |
| Body mass index, kg/m2 | 29.9 (3.5) | 29.7 (3.2) | 29.5 (3.8) | .708 |
| Hypertension | 103 (98.1) | 107 (99.1) | 108 (100) | .759 |
| Dyslipidemia | 74 (70.5) | 77 (71.3) | 72 (66.7) | .733 |
| Diabetes type 2 | 23 (21.9) | 29 (26.9) | 22 (20.4) | .498 |
| Chronic ischemic heart disease | 2 (1.9) | 0 | 1 (0.9) | .765 |
| Antihypertensive drugs | ||||
| ACEi | 40 (38.1) | 46 (42.6) | 40 (37.0) | .674 |
| ARB | 22 (21.0) | 23 (21.3) | 21 (19.4) | .938 |
| Calcium antagonists | 23 (21.9) | 25 (23.1) | 13 (12.0) | .748 |
| Beta-blocker | 11 (10.5) | 16 (14.8) | 17 (15.7) | .492 |
| Diuretic | 19 (18.1) | 21 (19.4) | 23 (21.3) | .839 |
| Other | 2 (1.9) | 1 (0.9) | 2 (1.8) | .810 |
| Lipid-lowering drugs | ||||
| Statins | 41 (39.0) | 41 (38.0) | 35 (32.4) | .555 |
| Other | 5 (4.8) | 5 (4.6) | 5 (4.6) | .998 |
| Antiplatelets | ||||
| Aspirin | 13 (12.4) | 15 (13.9) | 22 (20.4) | .230 |
ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers.
Unless otherwise indicated, data are expressed as No. (%) or mean±standard deviation.
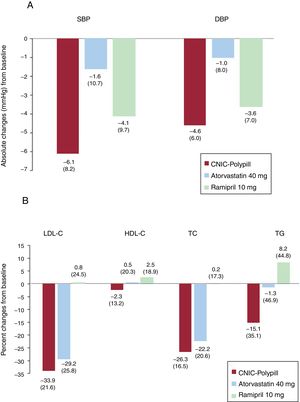
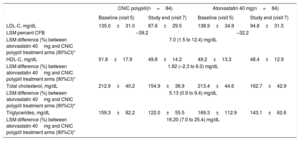
The absolute change from baseline in the adjusted mean 24-hour SBP in the analysis of covariance for participants between the ramipril 10mg and CNIC polypill treatment arms was 1.77mmHg (–0.5 to 4.0), thus making it possible to exclude clinically significant negative interactions between ramipril and other components of the CNIC polypill. The results showed a tendency toward a greater reduction in SBP (1.77mmHg) in the CNIC polypill group compared with ramipril alone (table 2). The change from baseline in the adjusted mean 24-hour DBP for participants between ramipril 10mg and the CNIC polypill treatment arms was 0.79 (–0.8 to 2.4). The absolute changes in SBP and DPB at the end of the study is shown in figure 3.
Changes in blood pressure
| CNIC polypill(n=84) | Ramipril 10 mg(n=73) | |||
|---|---|---|---|---|
| Baseline (visit 3) | Study end (visit 7) | Baseline (visit 3) | Study end (visit 7) | |
| SBP, mmHga | 139.3±12.5 | 133.2±12.9 | 138.1±11.9 | 134.0±12.8 |
| CFB LSM | –6.0 | –4.2 | ||
| Ratio of LSM (90%CI) between CNIC polypill and ramipril 10mg treatment arms | 1.42 (0.9 to 2.0) | |||
| CFB LSM difference in the adjusted mean 24-h SBP for participants between ramipril 10mg and CNIC polypill treatment arms (90%CI)b | 1.77 (–0.5 to 4.0) mmHg | |||
| DBP, mmHg | 83.6 ± 9.7 | 79.0 ± 7.0 | 82.8 ± 9.1 | 79.3 ± 9.3 |
| CFB LSM difference in the adjusted mean 24-h DBP for participants between ramipril 10mg and CNIC polypill treatment arms (90%CI) | 0.79 (–0.8 to 2.4) mmHg | |||
90%CI, 90% confidence interval; CFB, change from baseline; DBP, diastolic blood pressure; LSM, least square mean; SBP, systolic blood pressure.
Absolute changes (least square mean [LSM], standard deviation [SD]) in SBP/DBP (panel A) and percentage changes in lipid parameters (panel B) from baseline for the CNIC polypill (n=84), atorvastatin 40mg (n=84), and ramipril 10mg (n=73). DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
The mean±standard deviation percentage change in LDL-C from baseline was 33.9±21.6 and 29.2±25.8 in the CNIC polypill and atorvastatin arms, respectively. The LDL-C least square means difference in the percent change from baseline to the end of treatment was higher 7.0 [1.5–12.4]) in the polypill arm, suggesting a synergistic effect between the 2 components (ramipril and atorvastatin) in the CNIC polypill. Reductions in total cholesterol and triglycerides were also higher in the polypill arm, thus suggesting a synergistic effect between the polypill components (least square means difference of 5.13 [0.9–9.4] and 16.20 [7.0–25.4], respectively). The difference in HDL-C between groups was smaller (1.82 [–2.3 to 6.0]) (table 3). The percentage change in lipid parameters at the end of the study is shown in figure 3. The results of the analysis for intention-to-treat were similar to those of the per protocol population.
Changes in lipid parameters
| CNIC polypill(n=84) | Atorvastatin 40 mg(n=84) | |||
|---|---|---|---|---|
| Baseline (visit 5) | Study end (visit 7) | Baseline (visit 5) | Study end (visit 7) | |
| LDL-C, mg/dL | 135.0±31.0 | 87.6±29.5 | 138.9±34.9 | 94.8±31.5 |
| LSM percent CFB | –39.2 | –32.2 | ||
| LSM difference (%) between atorvastatin 40mg and CNIC polypill treatment arms (90%CI)* | 7.0 (1.5 to 12.4) mg/dL | |||
| HDL-C, mg/dL | 51.8±17.9 | 49.8±14.2 | 49.2±13.3 | 48.4±12.9 |
| LSM difference (%) between atorvastatin 40mg and CNIC polypill treatment arms (90%CI)* | 1.82 (–2.3 to 6.0) mg/dL | |||
| Total cholesterol, mg/dL | 212.9±40.2 | 154.9±36.9 | 213.4±44.6 | 162.7±42.9 |
| LSM difference (%) between atorvastatin 40mg and CNIC polypill treatment arms (90%CI)* | 5.13 (0.9 to 9.4) mg/dL | |||
| Triglycerides, mg/dL | 159.3±82.2 | 122.0±55.5 | 169.3±112.9 | 143.1±62.6 |
| LSM difference (%) between atorvastatin 40mg and CNIC polypill treatment arms (90%CI)* | 16.20 (7.0 to 25.4) mg/dL | |||
90%CI, 90% confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LSM, least square mean.
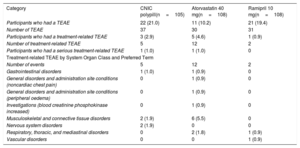
With regard to safety, overall, 9 of 321 participants (2.8%) experienced a total of 19 treatment-related treatment-emergent adverse events (TEAEs) after randomization. Table 4 shows the overall adverse event during the randomization phase in the safety population (safety population is defined as all randomized patients who received at least 1 dose of the study drugs). After randomization, the number of participants with treatment-related TEAEs was higher in participants in the atorvastatin 40mg arm (12; 11.1%) than in the combination pill treatment arm (5; 4.7%) and ramipril 10mg treatment arm (2; 1.8%). Most TEAEs were mild in intensity. No relevant findings were reported for laboratory parameters, vital signs, physical examination, or electrocardiogram. No deaths were reported during the study.
Overall adverse events during the randomization phase in the safety population
| Category | CNIC polypill(n=105) | Atorvastatin 40 mg(n=108) | Ramipril 10 mg(n=108) |
|---|---|---|---|
| Participants who had a TEAE | 22 (21.0) | 11 (10.2) | 21 (19.4) |
| Number of TEAE | 37 | 30 | 31 |
| Participants who had a treatment-related TEAE | 3 (2.9) | 5 (4.6) | 1 (0.9) |
| Number of treatment-related TEAE | 5 | 12 | 2 |
| Participants who had a serious treatment-related TEAE | 1 (1.0) | 1 (1.0) | 0 |
| Treatment-related TEAE by System Organ Class and Preferred Term | |||
| Number of events | 5 | 12 | 2 |
| Gastrointestinal disorders | 1 (1.0) | 1 (0.9) | 0 |
| General disorders and administration site conditions (noncardiac chest pain) | 0 | 1 (0.9) | 0 |
| General disorders and administration site conditions (peripheral oedema) | 0 | 1 (0.9) | 0 |
| Investigations (blood creatinine phosphokinase increased) | 0 | 1 (0.9) | 0 |
| Musculoskeletal and connective tissue disorders | 2 (1.9) | 6 (5.5) | 0 |
| Nervous system disorders | 2 (1.9) | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 0 | 2 (1.8) | 1 (0.9) |
| Vascular disorders | 0 | 0 | 1 (0.9) |
TEAE, treatment-emergent adverse event.
TEAE included participants who used the study medication. Percentages were based on all participants who were dispensed medication in each treatment arm for the actual treatment received.
Unless otherwise indicated, data are expressed as No. (%) or mean±standard deviation.
The results of our study suggest that a negative pharmacodynamic interaction between the components of the polypill can be ruled out (ie, a reduction in the BP-lowering effect of ramipril owing to a possible interaction with aspirin). Furthermore, no significant differences were observed in the total number of participants experiencing TEAEs in the CNIC polypill group compared with the ramipril or atorvastatin group. Therefore, the CNIC polypill should be considered at least as safe as the reference drugs when administered alone.
Although the results of a previous pharmacokinetic study18 showed bioequivalence between the polypill and the corresponding monocomponents when administered alone, the results of the pharmacodynamic interaction study have not been previously reported. The potential negative effect on the BP-lowering effect was hypothesized to be associated with the different mechanisms of action of both components (ACEi and aspirin) in the production of prostaglandins. On the one hand, ACEi increase bradykinin levels, thus promoting the production of vasodilatory prostaglandins; on the other, aspirin inhibits prostaglandin production through its effect on cyclo-oxygenase activity.19 Although this negative association has not been confirmed by some authors,20 others have suggested a dose-related effect with medium to high doses, but not with low doses of aspirin administered concomitantly with ACEi.21–23 We observed no negative pharmacodynamic interaction in our study, although a tendency was observed toward a more marked reduction in SBP in the CNIC polypill group than in the group that received ramipril 10mg alone. Of note, after the ramipril run-in phase, the SBP decrease was lower in the CNIC group than in the ramipril group. These discrepancies could be explained by the different measurement techniques used during the study. Finally, 2 meta-analyses conducted to evaluate the impact of this interaction ruled out the possibility that concomitant use of the 2 medicines had a negative impact on their protective cardiovascular capacity.24,25
Our results also showed a more marked reduction in LDL-C, total cholesterol, and triglycerides in the CNIC polypill arm compared with atorvastatin 40mg, which was independent of medication adherence, as the analysis was performed in the per protocol population. To the best of our knowledge, this is the first study showing a possible synergistic effect of an ACEi and a statin within a polypill in terms of LDL-C. Although there are enough data demonstrating that the combination of a statin with ACEi has a positive synergic effect on the cardiovascular protective activity (ie, beneficial effects on endothelial function, vascular inflammation, insulin resistance, and the development, progression, and rupture of atheromatous plaques29–33), few data are available on the effects of ACEi on LDL-C levels. Several studies showed that the combination of a statin with certain ACEis decrease the levels of LDL-C,32,33 while found no effect on these parameters.34,35 It has been demonstrated that angiotensin II up-regulates Niemann-Pick C1-like 1 proteine (NPC1L1 protein, identified as a key molecule of cholesterol absorption in the intestine) mRNA and protein levels in Caco-2 cells, which were completely blocked by an angiotensin II type 1 receptor blocker.36 This action could be related to the effect observed in our study. However, the present study was designed to test the pharmacodynamic equivalence of the CNIC polypill with the monocomponent, but not the mechanism of action underlying the observed synergistic action. Thus, further studies are needed to clarify the mechanism of action of this interesting finding. Finally, the difference in LDL-C levels in the CNIC arm regarding the other groups was 7%. It is well established that each doubling of a statin dose reduces LDL-C by about 6%.26–28 Thus, the present results suggest that the use of a polypill containing atorvastatin, aspirin, and ramipril would be equivalent to doubling the dose of a statin in monotherapy in terms of the reduction in LDL-C, although a specific study should be carried out to evaluate this equivalence.
This potential synergistic effect could also be translated into a relevant clinical effect, given the strong relationship between reduced LDL-C and the decrease in the frequency of cardiovascular events. Thus, it has been demonstrated that every 1-mmol/L reduction in LDL-C corresponds to a 20% reduction in deaths due to coronary heart disease.37
Finally, there were no differences between the CNIC polypill group and the corresponding monotherapies in the total number of TEAEs. Therefore, the possible synergistic effect between atorvastatin and ramipril does not translate into a higher risk of adverse effects.10,13
LimitationsThis study has some limitations. The fact that it was not a double-blind study and its open-label design could have some impact on the results. In addition, 20% of randomized patients were not included in the final analysis, which could also be considered as another limitation of the study, although the main reason for this effect was related to protocol violations and not to a lack to treatment adherence. Moreover, the final per protocol population was as expected. These findings could minimize the above-mentioned limitation. A randomized double-blind study is needed to confirm not only the synergistic effects of the CNIC polypill on cardiovascular risk factors, but also on the reduction in the frequency of cardiovascular events.
CONCLUSIONSThe results of this study rule out a negative effect on BP resulting from the interaction between the components of the CNIC polypill. The reduction in LDL-C was greater in the CNIC polypill group, thus suggesting a synergistic effect of the components. This synergistic effect did not translate into more frequent adverse effects.
- -
Several clinical trials have demonstrated the safety and efficacy of cardiovascular polypills in cardiovascular prevention.
- -
The CNIC polypill contains aspirin, ramipril and atorvastatin and, while bioequivalence between the polypill and the 3 drugs given alone has been demonstrated, no studies have assessed the possible pharmacodynamic interactions.
- -
However, this information is important, given the conflictive data on the potential interaction between the monocomponents when administered separately.
- -
The results of this study rule out a negative effect on blood pressure resulting from the interaction between the components of the CNIC polypill.
- -
The reduction in LDL-C was greater in the CNIC polypill group, suggesting a synergistic effect of the components.
- -
Therefore, no differences in clinical effectiveness or safety can be expected when the monocomponents are administered as a polypill.
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain) with funding from Ferrer Internacional S.A.
CONFLICTS OF INTERESTJ.R. González-Juanatey has received speaker fees Ferrer. N. Oudovenko is a Ferrer employee. The other authors have nothing to disclose.
Authors would like to thank José Luis Lorenzo Ferrer, an employee of Ferrer, who helped to edit the manuscript.




![Absolute changes (least square mean [LSM], standard deviation [SD]) in SBP/DBP (panel A) and percentage changes in lipid parameters (panel B) from baseline for the CNIC polypill (n=84), atorvastatin 40mg (n=84), and ramipril 10mg (n=73). DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides. Absolute changes (least square mean [LSM], standard deviation [SD]) in SBP/DBP (panel A) and percentage changes in lipid parameters (panel B) from baseline for the CNIC polypill (n=84), atorvastatin 40mg (n=84), and ramipril 10mg (n=73). DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.](https://static.elsevier.es/multimedia/18855857/0000007400000001/v1_202012200625/S1885585719304153/v1_202012200625/en/main.assets/thumbnail/gr3.jpeg?xkr=eyJpdiI6IjFMeGRQU2RJdVJpVXlOU3BUZmVVamc9PSIsInZhbHVlIjoiMWoxTzRXcmExRmxQd21Kd0d3SWx5cGdYVExGTk1pMFpLcCtzdko0OHBHYz0iLCJtYWMiOiIyMjVlYzM4NjFmZDYxYmQ1NzgyODRjZGZiZWUwYTJjYWQyMGJkNzc4NDg0ODQ2NzZmMmIxZjdmYTdmZjc3NDBhIiwidGFnIjoiIn0=)