It is well established that high on-treatment platelet reactivity to adenosine diphosphate during clopidogrel therapy is an independent risk factor for ischemic event occurrences in a postpercutaneous coronary intervention patients. However, the precise role of platelet function testing remains debated. Platelet function testing to ensure optimal platelet inhibition has been recommended by some authorities to improve outcomes in patients treated with clopidogrel. Recent prospective, randomized trials of personalized antiplatelet therapy have failed to demonstrate a benefit of platelet function testing in improving outcomes. In this review article, we discuss the mechanisms responsible for clopidogrel nonreponsiveness, recent trials of platelet function testing, and other new developments in the field of personalized antiplatelet therapy.
Keywords
Adenosine diphosphate (ADP)-P2Y12 receptor interaction plays a pivotal role in platelet-rich thrombus generation at sites of plaque rupture and subsequent ischemic event occurrence in patients with coronary artery disease. The clinical efficacy of dual antiplatelet therapy consisting of acetylsalicylic acid and a P2Y12 receptor blocker has been demonstrated in a wide range of high-risk coronary artery disease patients.1 However, clopidogrel therapy, the most widely used P2Y12 receptor blocker, is associated with widely variable pharmacodynamic response and approximately 1 in 3 clopidogrel-treated patients will have high on-treatment platelet reactivity (HPR). This complication has been strongly linked to postpercutaneous coronary intervention (PCI) ischemic event occurrence in observational studies of thousands of patients. Despite the fundamental importance of unblocked P2Y12 receptors in the genesis of thrombosis, the clear demonstration of clopidogrel nonresponsiveness, and even the identification of genes associated with resistance—CYP2C19*2 and *3—and their strong link to increased post-PCI ischemic risk, cardiologists do not usually determine platelet function or genetic polymorphisms in their high risk patients treated with clopidogrel. Coompared with the objective assessments and adjustments frequently made during treatment with most other cardiovascular drugs, this “nonselective” or “one-size-fits all” approach to clopidogrel, the most widely used P2Y12 inhibitor to prevent a catastrophic thrombotic event occurrence, is paradoxical.2,3
There has been long-term reluctance to assess platelet function due to the potential introduction of artifacts by laboratory methods, incomplete reflection of the actual in vivo thrombotic process, and failure to unequivocally establish a causal relation between the results of the test and thrombotic event occurrence. In the last decade, understanding of platelet receptor physiology has markedly improved, more potent P2Y12 receptor blockers that can overcome some of the limitations of clopidogrel have been developed, and cheaper generic clopidogrel is available. The introduction of more user-friendly platelet function assays that can reliably determine the antiplatelet effect of P2Y12 receptor blockers and point-of-care genetic assay that can readily determine genetic polymorphisms associated with the metabolism of P2Y12 receptor blockers (particularly clopidogrel and prasugrel) have stimulated strong interest in antiplatelet therapy monitoring and personalized antiplatelet therapy.3,4
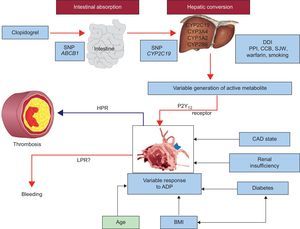
MECHANISMS RESPONSIBLE FOR CLOPIDOGREL NONREPONSIVENESSMultiple lines of evidence strongly suggest that variable and insufficient active metabolite generation are the primary explanations for clopidogrel response variability and nonresponsiveness where negligible or no antiplatelet effect of clopidogrel is observed.5 Variable levels of active metabolite generation following clopidogrel administration could be explained by: a) variable or limited intestinal absorption that may be influenced by ABCB1 gene polymorphism, and b) functional variability in CYP (cytochrome P450) isoenzyme activity that is influenced by drug-drug interactions and single nucleotide polymorphisms in genes encoding CYP isoenzymes.5
Numerous studies have evaluated the influence of single nucleotide polymorphisms of the gene encoding CY2C19 as well as single nucleotide polymorphisms of the p-glycoprotein transporter (ABCB1) gene on clopidogrel response variability and clinical outcomes.5 The most widely analyzed and most frequent single nucleotide polymorphisms are CYP2C19*2 (loss-of function [LoF] allele), which is associated with complete absence of enzyme activity, and *17 (gain-of-function allele), which is associated with increased expression and increased enzymatic activity.6 Less exposure to plasma clopidogrel active metabolite (32% relative reduction; P < .001) and less platelet inhibition (9% absolute reduction from baseline; P < .001) were demonstrated in healthy carriers of at least 1 CY2C19 LoF allele compared with noncarriers.7 In the first genome-wide association study, conducted in healthy Amish subjects, CYP2C19*2 was the only single nucleotide polymorphism associated with clopidogrel response variability and accounted for only 12% of the variation in platelet aggregation to ADP after clopidogrel treatment. In a replication study of PCI patients, carriers of the CYP2C19*2 allele had a ∼ 2.4-fold higher cardiovascular event rate than noncarriers.8 In a collaborative meta-analysis of various clinical trials primarily involving patients who underwent PCI (91%, 55% had acute coronary syndrome [ACS]), there was an increased risk of the composite end point occurrence of cardiovascular death, myocardial infarction or stroke among carriers of 1 LoF allele (hazard ratio [HR] = 1.55; 95% confidence interval [95%CI], 1.10–2.17; P = .01), as well as among carriers of 2 LoF alleles (HR = 1.76; 95%CI, 1.24–2.50; P = .002), compared with noncarriers. A significantly increased risk of stent thrombosis was observed in both carriers of 1 LoF allele (HR = 2.67; 95% CI, 1.69–4.22; P < .0001 and) and 2 LoF alleles (HR = 3.97; 95%CI, 1.75-9.02; P = .001) than in noncarriers.9
Subsequent retrospective analyses of trials involving non-PCI patients failed to demonstrate a significant association between CYP2C19 LoF allele carriage and adverse clinical outcomes. The relation of the gain of function allele (CYP2C19*17) carrier status, and ABCB1 and paraoxonase-1 genotypes to antiplatelet response and clinical outcomes in clopidogrel-treated patients are inconclusive at this time.9–12 In addition, LoF allele carrier status is an important independent predictor of the pharmacodynamic response to clopidogrel and the outcomes of high-risk clopidogrel-treated patients who have undergone PCI. In 2009, the Food and Drug Administration noted that healthcare professionals should be aware that tests are available to determine genotype and that the antiplatelet response in poor metabolizers is increased by high-dose clopidogrel. The Food and Drug Administration also recommended the use of other antiplatelet medications or alternative dosing strategies for clopidogrel in poor metabolizers.13
Finally, it should be noted that the CYP2C19 isoenzyme is not the only factor determining the antiplatelet response to clopidogrel, as even in poor metabolizers, some degree of platelet inhibition has been observed when no enzyme activity is expected. In a study of healthy persons with homozygous CYP2C19 extensive metabolizer genotype, clopidogrel 75mg/day was administered for 9 days. In this study, all identified factors together accounted for only 18% of interindividual variation in pharmacokinetic parameters and 32% to 64% of interindividual variation in platelet function as measured by VASP-P (vasodilator-stimulated phosphoprotein phosphorylation) assay, VerifyNow P2Y12 assay, and ADP-induced platelet aggregation by conventional assay.14 Stimulation of CYP3A4 activity by rifampin and St. Johns Wort, and CYP1A2 activity by tobacco smoking have been shown to enhance platelet inhibition induced by clopidogrel.15–17 The effect of smoking on the antiplatelet effect of clopidogrel has been associated with clinical outcomes and may, in part explain the “smoker's paradox”.18 Conversely, agents that compete with clopidogrel for CYP and/or inhibit CYP, attenuate the antiplatelet effect of clopidogrel. A diminished pharmacodynamic response to clopidogrel has been observed with coadministration of proton pump inhibitors such as omeprazole, lipophilic statins, and calcium channel blockers that are metabolized by the CYP2C19 and CYP3A4 isoenzymes.19–21 Although a diminished level of platelet inhibition induced by clopidogrel has been demonstrated in some ex vivo studies following coadministration of these agents, the effect of these interactions on the risk of ischemic event occurrence remains controversial. In addition to the above mechanisms explaining clopidogrel pharmacodynamic variability, old age, increased body mass index, renal insufficiency, diabetes mellitus, and ACS have also been associated with a diminished antiplatelet response to clopidogrel (Figure).22 Finally, noncompliance is an obvious factor that must be excluded in the diagnosis of clopidogrel nonresponsiveness. When attempting to define causality for high platelet reactivity related to the occurrence of clinical events in patients receiving clopidogrel, all of the aforementioned mechanisms should be considered. The advantages and disadvantages of platelet function testing (PFT) and genotyping are given in Table 1.
Various factors influencing platelet reactivity and clinical events during clopidogrel therapy. ADP, adenosine diphosphate; BMI, body mass index; CAD, coronary artery disease; CCB, calcium channel blocker; CYP, cytochrome P450; DDI, drug-drug interaction; HPR, high on-treatment platelet reactivity; LPR, low on-treatment platelet reactivity. PON 1, paraoxonase 1; PPI, proton pump inhibitor; SJW, St. John's wort; SNP, single nucleotide polymorphism. Adapted with permission from Gurbel et al.22.
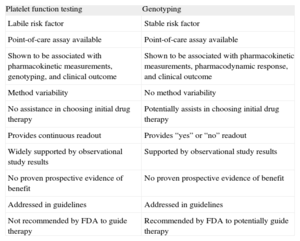
Advantages and Disadvantages of Evaluating Platelet Function Testing and Genotyping in Patients Treated With P2Y12 Receptor Blockers
| Platelet function testing | Genotyping |
| Labile risk factor | Stable risk factor |
| Point-of-care assay available | Point-of-care assay available |
| Shown to be associated with pharmacokinetic measurements, genotyping, and clinical outcome | Shown to be associated with pharmacokinetic measurements, pharmacodynamic response, and clinical outcome |
| Method variability | No method variability |
| No assistance in choosing initial drug therapy | Potentially assists in choosing initial drug therapy |
| Provides continuous readout | Provides “yes” or “no” readout |
| Widely supported by observational study results | Supported by observational study results |
| No proven prospective evidence of benefit | No proven prospective evidence of benefit |
| Addressed in guidelines | Addressed in guidelines |
| Not recommended by FDA to guide therapy | Recommended by FDA to potentially guide therapy |
FDA, Food and Drug Administration.
Based on the vast amount of accrued observational data, the recent 2011 American and European guidelines have given a class IIb recommendation in the high-risk patient for PFT or genotyping if the results of testing could alter management (Table 2).23–25 However, recent prospective randomized trials of PFT did not demonstrate a clinical benefit, thus questioning the utility of current PFT assays in antiplatelet therapy modification to influence outcomes.26–28
American and European Guidelines for Platelet Function Testing
| 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention23 |
| Class IIb |
| 1. PFT may be considered in patients at high risk for poor clinical outcomes (level of evidence: C) |
| 2. In patients treated with clopidogrel with high platelet reactivity, alternative agents, such as prasugrel or ticagrelor, might be considered (level of evidence: C) |
| Class III: no nenefit |
| 1. The routine clinical use of PFT to screen patients treated with clopidogrel who are undergoing PCI is not recommended (level of evidence: C) |
| 2012 ACCF/AHA focused update for the management of patients with UA/NSTEMI24 |
| Class IIb |
| 1. PFT to determine platelet inhibitory response in patients with UA/NSTEMI (or, after ACS and PCI) on P2Y12 receptor inhibitor therapy may be considered if the results of testing may alter management (level of evidence: B) |
| ESC guidelines for the management of ACS in patients presenting without persistent ST-segment elevation25 |
| Class IIb |
| 1. Genotyping and/or PFT may be considered in selected patients when clopidogrel is used (level of evidence: B) |
| 2. Increasing the maintenance dose of clopidogrel based on PFT is not advised as routine, but may be considered in selected patients (level of evidence: B) |
| 3. When it is considered appropriate to have a modest degree of P2Y12 inhibition at the time of surgery, such as is often the case early after an ACS in patients undergoing CABG surgery, then the drugs may be discontinued closer to the time of surgery. Under these circumstances, it is reasonable to stop clopidogrel 5 days before surgery, or less, if a validated PFT method shows a poor response to clopidogrel |
| 4. The routine clinical use of platelet function tests in clopidogrel-treated patients with ACS cannot be recommended |
ACS, acute coronary syndrome; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; CABG, coronary artery bypass graft; NSTEMI, non—ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PFT, platelet function testing; SCAI, Society for Cardiovascular Angiography and Interventions; UA, unstable angina.
In the GRAVITAS trial,26 the first large-scale investigation of personalized antiplatelet therapy in the elective PCI patient, patients with HPR were randomized to either a 600 mg extra loading dose of clopidogrel given the day after stenting followed by 150mg/day (high-dose) therapy or 75 mg/day clopidogrel therapy for 6 months. High-dose clopidogrel treatment was ineffective in reducing composite ischemic event occurrence and there was an unexpectedly low event rate (2.3%) in both groups.26 Potential explanations for this neutral observation include: a) suboptimal effectiveness of high-dose clopidogrel to overcome HPR.29 High-dose clopidogrel reduced the prevalence of HPR at 30 days in only 60% of patients. In support of this hypothesis, in the ELEVATE-TIMI 56 trial,30 a clopidogrel dose of up to 225mg/day was required to overcome the HPR; b) the cutoff for HPR may have been too high. In a time-dependent covariate Cox regression analysis of on-treatment platelet reactivity in GRAVITAS, P2Y12 reaction units (PRU) > 208 was an independent predictor of event-free survival at 60 days (HR = 0.23; 95%CI, 0.05-0.98; P =.047) and strongly trended to be an independent predictor at 6 months (HR = 0.54; 95%CI, 0.28-1.04; P = .06),31 and c) The majority of patients enrolled in GRAVITAS were low risk patients with stable coronary artery disease. Only treatment with a highly effective remedy to overcome HPR would have had a chance to produce positive results given the very low event rate. It is also possible that the single test used may not have reliably reflected the effect of clopidogrel on ADP-induced clot formation in all individuals.
In the TRIGGER-PCI study conducted in stable elective PCI patients (non—ST-segment elevation acute myocardial infarction and ST-segment elevation myocardial infarction [STEMI] excluded), > 208 PRU was used as the HPR cut point. A 10 mg/day dose of prasugrel was used in the active arm, which was highly effective in reducing the prevalence of HPR; only ∼ 6% of patients had HPR after 90 days of prasugrel therapy. However, the study was terminated early because of futility. There was only 1 occurrence of the primary end point among 236 patients who completed 6 months of follow-up. In addition, ∼ 30% of the enrolled patients declined randomization after being identified as having HPR.27
Finally, in the ARCTIC study,28 2440 patients scheduled for planned coronary stenting were randomly assigned to a strategy of platelet-function monitoring and drug adjustment, or to a conventional strategy without monitoring. In the monitoring arm, one third of patients had HPR (> 235 PRU) before stent implantation and 80% of these patients immediately received additional clopidogrel and 2.3% received a prasugrel loading dose. At the end of the study, 86% of patients in the conventional arm and 80% in the monitoring arm were on clopidogrel therapy and only 6% in the conventional arm and 12% in the monitoring arm were on prasugrel therapy. The 1-year primary composite end point of death, myocardial infarction, stent thrombosis, stroke, or urgent revascularization did not differ in the monitoring compared with the conventional arm (34.6% vs 31.1%, HR, 1.13; 95%CI, 0.98-1.29; P = .10). The main secondary end point, stent thrombosis or any urgent revascularization, and also the rate of major bleeding events did not differ significantly between groups.28 In this trial, the prevalence of ACS patients was low (27% non—ST-segment elevation ACS and 73% patients with stable coronary artery disease); patients at very high risk for early atherothrombotic events, eg, STEMI patients, were excluded from the study; prasugrel, a superior alternative to overcome HPR compared with double dose clopidogrel, was administered in only ∼ 10% of patients. Twice more patients were lost to follow up in the conventional arm than in the monitoring arm (3.8% vs 1.9%). Finally, the event rate in ARCTIC was mainly driven by periprocedural myocardial infarction that was assessed by nonuniform methodology postprocedure. The composite endpoint in this study also included other events that may not be related to platelet function.
While the results of the latter 3 randomized trials were negative, smaller studies have suggested that the PFT-directed approach may be effective with proper implementation. Two small multicenter trials employed the VASP-P assay to tailor incremental loading doses of clopidogrel in order to reduce on-treatment platelet reactivity below the HPR cutoff. This strategy was associated with significantly reduced subsequent adverse event occurrence, including early stent thrombosis without increasing TIMI (Thrombolysis In Myocardial Infarction) major or minor bleeding.32,33 Similarly, 2 other studies have suggested that the selective administration of a glycoprotein IIb/IIIa receptor inhibitor to patients undergoing elective PCI who were identified as poor responders to acetylsalicylic acid or clopidogrel was effective in reducing both 30-day and 1-year post-PCI ischemic events without increasing bleeding rates.34,35 Interestingly, all these studies aimed to decrease platelet reactivity below the threshold of HPR, which is associated with post-PCI ischemic events.
In the recent multinational prospective registry ADAPT-DES,36,37 platelet reactivity was assessed in 8583 patients (52% ACS patients) after successful PCI using VerifyNow point-of-care assays and the primary end point was 1 year definite or probable stent thrombosis. This study reinforced the independent association between HPR and definite/probable stent thrombosis. In this study, HPR (> 208 PRU) was independently associated with 30-day definite/probable stent thrombosis (HR = 3.0; P = .005), 1-year definite/probable stent thrombosis (HR = 2.49; P = .001) and myocardial infarction (HR = 1.42; P = .01) and 2-year definite/probable stent thrombosis (adjusted HR = 1.84; P = .009) and myocardial infarction (HR = 1.33; P =.01). In addition > 208 PRU was independently associated with a lower incidence of bleeding at 1 year (HR = 0.73; P = .002) and at 2 years (HR = 0.82; P = .02). An association between low on-treatment platelet reactivity to ADP and higher risk of bleeding has been demonstrated in recent studies.36,37
Although a major determinant of post-PCI thrombotic event occurrence, HPR is not the sole factor responsible for these events. In contrast, the absence of HPR is the best reassurance thus far for a low likelihood of future ischemic events. The HPR cutoff values reported in many studies are associated with high negative predictive values and low positive predictive values. However, given the overall low prevalence of thrombotic events in these studies, the low positive predictive values and high negative predictive values are understandable. Other factors, including demographic, clinical, and angiographic factors, must be taken into consideration to optimally identify the patients at greatest risk. Along this line, recent studies have suggested that adding clinical variables and genotype to platelet reactivity measurements (a combined risk factor) may improve risk prediction.38,39
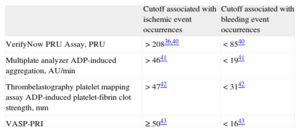
THERAPEUTIC WINDOW CONCEPTIn addition to the upper threshold for ischemic risk (ie, HPR), small translational research studies have demonstrated the relation of low platelet reactivity with bleeding. The concept of a “therapeutic window” of P2Y12 receptor reactivity associated with both ischemic event occurrence (upper threshold) and bleeding risk (lower threshold) has been proposed. A consensus document highlighting the above observations with a therapeutic window concept with updated cutoff for HPR and low platelet reactivity for P2Y12 inhibitor therapy has been published (Table 3).36,40–43 This approach is more meaningful while titrating the dose of more potent P2Y12 receptor blockers that are known to be associated with increased incidences of bleeding.2
Platelet Reactivity Cutoff Associated With Ischemic and Bleeding Events (Therapeutic Window)
| Cutoff associated with ischemic event occurrences | Cutoff associated with bleeding event occurrences | |
| VerifyNow PRU Assay, PRU | > 20836,40 | < 8540 |
| Multiplate analyzer ADP-induced aggregation, AU/min | > 4641 | < 1941 |
| Thrombelastography platelet mapping assay ADP-induced platelet-fibrin clot strength, mm | > 4742 | < 3142 |
| VASP-PRI | ≥ 5043 | < 1643 |
ADP, adenosine diphosphate; AU, aggregation units; PRU, P2Y12 reaction units; VASP-PRI, vasodilator-stimulated phosphoprotein-platelet reactivity index.
There has been a significant increase in the use of inexpensive generic clopidogrel. However, some important concerns have been raised regarding its widespread adaptation in the absence of rigorous studies demonstrating pharmacodynamic and clinical efficacy and safety. In addition to CYP2C19 LoF allele carriage, many epigenetic factors, including concomitant medications, influence clopidogrel metabolism and the resulting antiplatelet response that impact may clinical efficacy.4 As per the Abbreviated New Drug Application process for approval by the Food and Drug Administration, clinical trials are not required to prove the efficacy and safety of generic formulations. Generic formulations are approved based on small studies (eg, only pharmacokinetic measurements were performed periodically after a single 150mg clopidogrel dose over 32h in 24 healthy volunteers for the approval of one formulation of clopidogrel bisulfate.44 Concerns have been raised about the approval process adopted by the Food and Drug Administration in recommending generic forms of clopidogrel in the United States.44 In a recent study involving > 1500 ACS patients in Italy, generic formulation (clopidogrel base) compared with Plavix® was associated with greater ADP-induced platelet aggregation (average difference, 2%-11%) and a higher prevalence of HPR (42.4% vs 25.4%; P < .0001).45 However, other studies have demonstrated no significant differences between various generic formulations and Plavix®. However, there are significant limitations in the latter studies: only the difference in mean platelet aggregation was reported, measurements of active metabolite generation were lacking and, in most cases, only 1 laboratory method to assess pharmacodynamic response was used.46–49 Most recently, a 3.2-fold increase in the 30-day stent thrombosis rate with generic clopidogrel therapy compared with 3-year historic data on Plavix® (0.38% [4 of 1054] vs. 0.12% [17 of 14 432]; P = .050) has been reported in the United States.50 Some generic clopidogrel formulations have been reported to contain methyl chloride, which is known to exhibit genotoxic properties.51 These observations highlighted the concerns associated with widespread transition to generic clopidogrel and call for more precaution with close monitoring of clopidogrel response during the adoption of generic clopidogrel.
P2Y12 RECEPTOR BLOCKER THERAPY IN ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION PATIENTSAn accumulating body of data suggests that drug absorption in ACS patients is impaired, particularly in those with STEMI. Impaired bioavailability of clopidogrel has been demonstrated in STEMI patients, resulting in suboptimal platelet inhibition compared with healthy controls.52 In a recent prospective, single-blind study, 55 STEMI patients undergoing PCI were randomized to either ticagrelor or prasugrel and serial PFT was performed.53 Although platelet reactivity measured by VerifyNow was high compared with previous studies conducted in stable, non-PCI patients at 1 h, it did not differ significantly between ticagrelor and prasugrel therapy. However, HPR at 2h persisted in a significant percentage of patients in both groups and again differed from the findings in stable, non-PCI patients who showed a negligible frequency of HPR.53 In another study of 50 patients with STEMI undergoing primary PCI on bivalirudin monotherapy, patients were randomly treated with 60mg prasugrel loading dose or 180mg ticagrelor loading dose. Both prasugrel and ticagrelor therapy were effective in inhibiting platelet reactivity in only ∼ 50% of the patients at 2h. At least 4h were required to achieve effective platelet inhibition in ∼ 80% of the patients. Interestingly, morphine use was associated with a delayed activity of both agents.54 These findings in STEMI patients are highly thought-provoking and future larger studies are needed to confirm them and their clinical relevance. In a subsequent study, 24 healthy volunteers were randomly treated with placebo or 5mg intravenous morphine in addition to 600mg clopidogrel. In this study, morphine use was associated with delayed clopidogrel absorption and reduced clopidogrel active metabolite levels, which were accompanied by delayed maximum platelet inhibition (up to 4h).55
PERSONALIZED ANTIPLATELET THERAPY IN THE SURGICAL PATIENTThe major rationale for 5- to 7-day discontinuation of P2Y12 receptor inhibitor treatment recommended by the guidelines in patients undergoing coronary artery bypass grafting was to allow platelet function recovery, thereby avoiding excessive perioperative bleeding.56–59 A recent study demonstrated that clopidogrel-treated patients undergoing first time on-pump coronary artery bypass grafting had the same perioperative bleeding as clopidogrel-naïve patients when their surgery was timed on the basis of a preoperative assessment of platelet reactivity. Preoperative platelet reactivity was measured by thrombelastography with platelet mapping. Surgery in patients treated with clopidogrel was scheduled within 24h of the last dose of clopidogrel in those with a maximum amplitude (MAADP) > 50mm, within 3 days to 5 days of the last dose in those with an MAADP 35 mm to 50mm, and 5 days after the last dose in those with an MAADP < 35mm.57 In the 2012 Society of Thoracic Surgeons guidelines there is a class IIa recommendation for PFT in clopidogrel-treated patients to shorten the wait time (Table 4).60
2012 Society of Thoracic Surgeons Guideline for Platelet Function Testing in Patients Having Cardiac and Noncardiac Operations60
| Treatment options for patients on antiplatelet drugs who require urgent operations |
| Class IIa |
| For patients on dual antiplatelet therapy, it is reasonable to make decisions about surgical delay based on tests of platelet inhibition rather than arbitrary use of a specified period of surgical delay (level of evidence: B) |
| Monitoring platelet function |
| Class IIb |
| Because of their high negative predictive value, preoperative point-of-care testing to assess bleeding risk may be useful in identifying patients with high residual platelet reactivity after usual doses of antiplatelet drugs, and who can undergo operation without elevated bleeding risk (evel of evidence: B) |
| Point-of-care testing to assess perioperative platelet function may be useful in limiting blood transfusion (level of evidence: B) |
| Antiplatelet drugs after cardiac operations |
| Class IIb |
| Once postoperative bleeding risk is decreased, testing of response to antiplatelet drugs, either with genetic testing or with point-of-care platelet function testing, early after cardiac procedures might be considered to optimize the antiplatelet drug effect and minimize thrombotic risk to vein grafts (level of evidence: B) |
| For patients with high platelet reactivity after usual doses of clopidogrel, it may be helpful to switch to another P2Y12 inhibitor (eg, prasugrel or ticagrelor) |
Currently, the evidence indicates that HPR and CYP2C19 LoF carriage are strongly associated with poorer clinical outcomes in high-risk clopidogrel-treated patients who have undergone PCI. It should be acknowledged that randomized trials of personalized antiplatelet therapy are associated with major limitations, such as the enrollment of low-risk patients, which resulted in low event rates—thus these studies were underpowered—and the use of high-dose clopidogrel, which is not an optimal strategy to overcome HPR and to improve clinical outcomes. Therefore, the results of these randomized trials should not be used to refute the utility of PFT or personalized antiplatelet therapy strategies.
Data from the TRITON-TIMI 38 and PLATO trials strongly suggest that prasugrel and ticagrelor are effective alternatives to clopidogrel that overcome the influence of the LoF allele carrier status. Pharmacodynamic studies demonstrate that prasugrel and ticagrelor are effective in overcoming HPR during clopidogrel therapy.41,42 Therefore, a reasonable strategy is to assess platelet function in high-risk clopidogrel-treated patients (eg, patients with current or prior ACS, a history of stent thrombosis and target vessel revascularization, poor left ventricular function, multivessel stenting, complex anatomy—bifurcation, long, small stents—high body mass index, diabetes mellitus, and patients cotreated with proton-pump inhibitors) and use more potent P2Y12 receptor therapy selectively in the patient with HPR. Furthermore, unselected therapy with the new P2Y12 receptor blockers is associated with increased bleeding. By personalizing therapy, clinicians can find the antiplatelet agent that achieves the optimal level of platelet reactivity for the patient, regardless of the cost. If generic clopidogrel is indeed pharmacodynamically effective in particular patients, offering them this less expensive option seems favorable from both cost and efficacy standpoints.
Finally, a future study demonstrating noninferiority from the selective use of generic clopidogrel and the new P2Y12 inhibitors may be more likely. However, low event rates in current practice would require enrollment of a very large number of patients and the prospect of finding funding for this type of endeavor is not promising. Thus, we must rely on the guidelines and the existing observational data while keeping fully in mind the role that platelet physiology plays in catastrophic event occurrence in PCI patients.
CONFLICTS OF INTERESTDr. Gurbel has acted as a consultant for Daiichi Sankyo, Eli-Lilly, Bayer, AstraZeneca, Accumetrics, Nanosphere, Sanofi-Aventis, Merck, Medtronic, CSL and Haemonetics; has previously received grants from or has pending grants with the National Institutes of Health, Daiichi Sankyo, Eli-Lilly, CSL, AstraZeneca, Haemonetics, Medtronic, Harvard Clinical Research Institute and Duke Clinical Research Institute; and has provided lectures/speakers bureaus services to Eli-Lilly, Daiichi Sankyo, Nanosphere, Sanofi-Aventis, Merck and Iverson Genetics. Dr. Gurbel holds stock or stock options in Merck, Medtronic, and Pfizer and holds patents in the area of personalized antiplatelet therapy and interventional cardiology.
Section sponsored by AstraZeneca