During the last few years, the number of patients receiving anticoagulant and antiplatelet therapy has increased worldwide. Since this is a chronic treatment, patients receiving it can be expected to need some kind of surgery or intervention during their lifetime that may require treatment discontinuation. The decision to withdraw antithrombotic therapy depends on the patient's thrombotic risk versus hemorrhagic risk. Assessment of both factors will show the precise management of anticoagulant and antiplatelet therapy in these scenarios. The aim of this consensus document, coordinated by the Cardiovascular Thrombosis Working Group of the Spanish Society of Cardiology, and endorsed by most of the Spanish scientific societies of clinical specialities that may play a role in the patient-health care process during the perioperative or periprocedural period, is to recommend some simple and practical guidelines with a view to homogenizing daily clinical practice.
Keywords
The number of patients on anticoagulant therapy has risen significantly in recent years. In Spain alone, over 800 000 patients are estimated to be taking anticoagulants, mainly for atrial fibrillation (AF).1 Use of antiplatelet drugs is also rising because these drugs are prescribed for the secondary prevention of atherosclerotic disease and because there has been a rise in the number of percutaneous coronary interventions (PCIs) and stent implantations.2–6 As most patients on antithrombotic therapy have chronic disease, at some point in their lives they will more than likely need surgery or an invasive procedure that requires temporary interruption of their anticoagulant or antiplatelet therapy.7 Decisions regarding interruptions in antiplatelet therapy are particularly important, as premature withdrawal has been linked to a higher risk of cardiovascular events, including stent thrombosis.8 The decision to continue or discontinue therapy prior to a a surgical intervention or invasive procedure should be taken following an evaluation of thrombotic and bleeding risk. In short, both anticoagulation and antiplatelet management strategies will be determined by the interaction between these 2 risk factors.5-9
Although numerous guidelines have been published on the perioperative management of antithrombotic therapy, their application to clinical practice is limited.7,9–17 Many of the publications address only some of the players involved in perioperative care (eg, surgeons or anesthesiologists), while others focus on specific areas of action (eg, endoscopic procedures) or have become obsolete following the appearance of new pharmacologic treatments. To address these shortcomings, a working group formed by members of the majority of Spanish scientific societies that represent professionals involved in perioperative or periprocedural care was created under the supervision of the Spanish Cardiology Society (Table 1). One of the outcomes of this group was the current consensus document, which is designed to provide a series of clear, practical recommendations on the management of antithrombotic drugs in patients undergoing surgery or other procedures that require the temporary interruption of anticoagulant or antiplatelet therapy. The ultimate aim of these recommendations, which are endorsed by each of the participating societies, is to help standardize clinical practice.
Scientific Societies That Participated in the Working Group and Endorse the Recommendations in This Consensus Document
| Spanish Society of Cardiology (SEC) | David Vivas, Inmaculada Roldán, Jose Luis Ferreiro, Francisco Marín, Vanessa Roldán, Antonio Tello-Montoliu, Juan Miguel Ruiz-Nodar, Juan José Gómez-Doblas, Manuel Anguita, and Andrés Íñiguez |
| Spanish Society of Anesthesiology, Reanimation and Pain Therapy (SEDAR) | Raquel Ferrandis, Juan Vicente Llau, Concepción Cassinello, Aurelio Gómez-Luque, Francisco Hidalgo, and Pilar Sierra |
| Spanish Society of Angiology and Vascular Surgery (SEACV) | María José Ramos-Gallo |
| Spanish Society of Thoracic-Cardiovascular Surgery (SECTCV) | Rafael Muñoz |
| Spanish Association of Surgeons (AEC) | Juan Ignacio Arcelus |
| Spanish Society of Plastic, Reconstructive and Cosmetic Surgery (SECPRE) | Francisco Leyva |
| Spanish Society of Digestive Disorders (SEPD) | Fernando Alberca |
| Spanish Society of Gynecology and Obstetrics (SEGO) | Raquel Oliva |
| Spanish Society of Hematology and Hemotherapy (SEHH) | Pascual Marco Vera |
| Spanish Society of Thrombosis and Hemostasis (SETH) | José Mateo Arranz |
| Spanish Society of Primary Care Physicians (SEMERGEN) | José Luis Listerri |
| Spanish Society of Family and Community Medicine (SEMFYC) | José María Lobos |
| Spanish Society of General and Family Physicians (SEMG) | Isabel Egocheaga and Vicente Palomo |
| Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC) | Ainhoa Serrano |
| Spanish Society of Internal Medicine (SEMI) | Olga Madridano |
| Spanish Society of Emergency Medicine (SEMES) | Alfonso Martín |
| Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) | Ana María Gómez and Carmen Montero |
| Spanish Society of Neurosurgery (SENEC) | Fuat Arikan and Luis Ley |
| Spanish Society of Ophthamology (SEO) | Enrique Santos-Bueso |
| Spanish Society of Periodontology (SEPA) | Elena Figuero, Antonio Bujaldón, and Adrián Guerrero |
| Spanish Society of Vascular and International Radiology (SERVEI) | José Urbano |
| Spanish Society of Traumatology and Orthopedics (SECOT) | Rafael Otero |
| Spanish Association of Urology (AEU) | Juan Francisco Hermida |
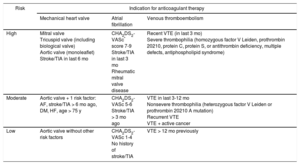
Thromboembolic risk associated with the indication for anticoagulant therapy (a mechanical heart valve, AF, or venous thromboembolism) is classified as high when the annual risk of arterial or venous thromboembolism is greater than 10%, moderate when it is between 5% and 10%, and low when it is less than 5%.18 Recent recommendations that use CHA2DS2-VASc rather than CHADS2 scores for stroke risk assessment were taken into account for patients with AF.16 The risk stratification for thromboembolism according to the indication for anticoagulant therapy is presented in Table 3.
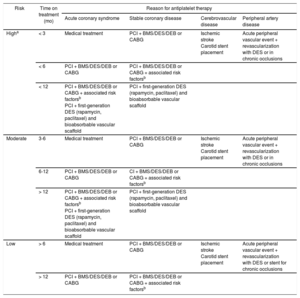
Stratification of Thrombotic Risk in Patients on Antiplatelet Therapy
| Risk | Time on treatment (mo) | Reason for antiplatelet therapy | |||
|---|---|---|---|---|---|
| Acute coronary syndrome | Stable coronary disease | Cerebrovascular disease | Peripheral artery disease | ||
| Higha | < 3 | Medical treatment | PCI + BMS/DES/DEB or CABG | Ischemic stroke Carotid stent placement | Acute peripheral vascular event + revascularization with DES or in chronic occlusions |
| < 6 | PCI + BMS/DES/DEB or CABG | PCI + BMS/DES/DEB or CABG + associated risk factorsb | |||
| < 12 | PCI + BMS/DES/DEB or CABG + associated risk factorsb PCI + first-generation DES (rapamycin, paclitaxel) and bioabsorbable vascular scaffold | PCI + first-generation DES (rapamycin, paclitaxel) and bioabsorbable vascular scaffold | |||
| Moderate | 3-6 | Medical treatment | PCI + BMS/DES/DEB or CABG | Ischemic stroke Carotid stent placement | Acute peripheral vascular event + revascularization with DES or in chronic occlusions |
| 6-12 | PCI + BMS/DES/DEB or CABG | CI + BMS/DES/DEB or CABG + associated risk factorsb | |||
| > 12 | PCI + BMS/DES/DEB or CABG + associated risk factorsb PCI + first-generation DES (rapamycin, paclitaxel) and bioabsorbable vascular scaffold | PCI + first-generation DES (rapamycin, paclitaxel) and bioabsorbable vascular scaffold | |||
| Low | > 6 | Medical treatment | PCI + BMS/DES/DEB or CABG | Ischemic stroke Carotid stent placement | Acute peripheral vascular event + revascularization with DES or stent for chronic occlusions |
| > 12 | PCI + BMS/DES/DEB or CABG | PCI + BMS/DES/DEB or CABG + associated risk factorsb | |||
BMS, bare metal stent; CABG, coronary artery bypass grafting; CRF, chronic renal failure; DEB, drug-eluting balloon; DES, drug-eluting stent; DM, diabetes mellitus; HF, heart failure; LVEF, left ventricle ejection fraction; TIA, transient ischemic attack.
The following aspects should be considered when defining thrombotic risk associated with atherosclerotic conditions that require antiplatelet therapy: time to intervention, disease presentation (acute vs stable), the patient's clinical characteristics, and the treatment received (PCI and stent [type], surgery, or medical treatment only).19 It is important to emphasize that these risks have not been evaluated in clinical trials and that the recommendations presented are based on observational and retrospective studies and consensus documents by other groups.13 Based on a combination of the above factors, risk of thrombosis in patients taking antiplatelet agents is classified as high, moderate, or low, as shown in Table 3.13,20
Time from the ischemic event to the intervention is the most important determinant of events related to the interruption of dual antiplatelet therapy (DAPT) prior to surgery or an invasive procedure.21 Recent findings indicate that patients who undergo emergency surgery or surgery within 6 months of stent placement have an increased risk of thrombosis.22 The recommendations of the Cardiovascular Thrombosis Working Group of the Spanish Cardiology Society on the optimal duration of DAPT in patients with atherothrombotic disease are summarized in Figure 1.23,24
Recommendations on the optimal duration of dual antiplatelet therapy for patients with atherothrombotic disease. A, aspirin; BMS, bare metal stent; BVS, bioabsorbable vascular scaffold; C, clopidogrel; CABG, coronary artery bypass grafting; DEB, drug-eluting balloon; DES, drug-eluting stent; P, prasugrel; PCI, percutaneous coronary intervention; T, ticagrelor. Adapted with permission from Gómez-Polo et al.23.
Stent type is another important factor when evaluating thrombotic risk. PCI with a first-generation drug-eluting stent (DES) vs a bare metal stent is associated with a higher incidence of late and very late stent thrombosis.25 Second-generation DESs, however, offer greater safety as they incorporate improved design features and polymer coatings. They have even been associated with a lower incidence of thrombosis than metal stents.26 In addition, the emergence of new platforms and PCIs with drug-eluting balloons is driving the search for devices that offer increased safety without compromising effectiveness.27 Although experience with bioabsorbable vascular scaffolds is still limited, several studies have reported a higher incidence of long-term stent thrombosis with their use.5,23 The recommendation in such cases thus is to maintain DAPT for at least 12 months. Finally, patients who have undergone coronary artery bypass grafting or noninvasive medical treatment have a much lower risk of complications, as the risk of stent thrombosis, which is frequently catastrophic, is eliminated.
Another important consideration is acute vs stable disease. Patients with acute coronary syndrome have a greater risk of thrombosis than those with stable coronary artery disease. Comorbidity should also be evaluated. Conditions such as diabetes mellitus, chronic kidney disease, severe left ventricular dysfunction, and a history of stroke and/or transient ischemic attack have traditionally been associated with an increased risk of thrombosis.26 Patients who have undergone a complex PCI (placement of long, multiple, or overlapping stents, bifurcation or left main stents, or stents in vessels < 2.5mm or saphenous vein grafts) should also be considered to have an increased risk of thrombosis.28
Surgical intervention in patients with a recent history of stroke is a risk factor for new events, particularly in the 30 days following the stroke and after discontinuation of antiplatelet therapy.29 Stent thrombosis associated with peripheral artery disease has mainly been described in the first month. The risk is greatest in patients with DESs or stents used to treat chronic occlusions. The recent guidelines on the diagnosis and treatment of peripheral arterial diseases recommend DAPT for at least 1 month in patients who have undergone stenting for lower-extremity peripheral artery disease, regardless of stent type.24
BLEEDING RISKBleeding risk, like thrombotic risk, is stratified into 3 levels according to the characteristics of the procedure the patient is to undergo.7,10,13,16 Procedures with a low bleeding risk are those in which adequate hemostasis can be achieved and in which bleeding would not jeopardize the patient's life, affect the outcome of surgery, or require transfusion. Procedures with a moderate bleeding risk, in turn, are those in which it may be difficult to secure hemostasis or in which bleeding would increase the likelihood of the need for a transfusion or a repeat operation. Finally, procedures with a high bleeding risk are those in which perioperative bleeding could place the patient's life at risk or compromise the outcome of surgery. The stratification of risk for nonsurgical procedures (interventional cardiology, endoscopies, bronchoscopies, dental procedures, or vascular and interventional radiology) is less clear, as it is often not possible to achieve adequate primary hemostasis or compression in such cases. We therefore recommend considering percentages of risk and classifying procedures with a risk of less than 1% as low-risk procedures and others as moderate-to-high-risk procedures. Another useful factor for assessing risk is the potential severity of bleeding, which will vary according to location or the likelihood of achieving adequate hemostasis. We have summarized the most common surgeries and procedures identified by each of the scientific societies according to risk of bleeding in Table 1 of the supplementary material.9,10,13,16,30
Although bleeding risk is mainly influenced by type of procedure, it can be increased by factors inherent to the patient and/or the environment.30 In general, these factors are already covered in risk assessment systems for common diseases, such as AF and acute coronary syndrome, and include age > 65 years, kidney/liver failure, and concomitant pharmacological treatment that can alter hemostasis (eg, combination of antiplatelet and anticoagulant therapy).31–33 Other factors are a history of spontaneous bleeding in the 3 months before the intervention or in previous interventions, thrombocytopenia and/or thrombopathy, and variations in the international normalized ratio in patients being treated with a vitamin K antagonist (VKA).34
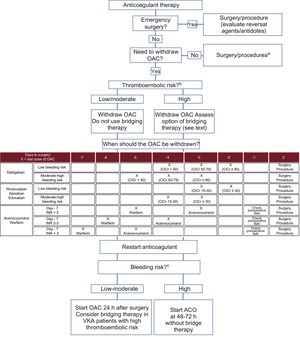
RECOMMENDATIONS FOR STOPPING AND RESTARTING ANTICOAGULANT THERAPYWe have established a series of simple recommendations to guide perioperative/periprocedural anticoagulation strategies according to the risk of thromboembolism and bleeding. These recommendations are summarized in the algorithm shown in Figure 2.9,16,30,34
Recommendations for interrupting and reintroducing oral anticoagulants according to type of intervention and the risk of thromboembolism and bleeding. CrCl, creatinine clearance (mL/min); INR, international normalized ratio; LMWH, low-molecular-weight heparin; OAC, oral anticoagulant; UFH, unfractionated heparin; VKA, vitamin K antagonist. aLow-risk procedures with an acceptable risk of very minor or clinically insignificant bleeding for the operator (Table 1 of the supplementary material). bClassification of thromboembolic risk (Table 2). cClassification of bleeding risk (Table 1 of the supplementary material).
In general, anticoagulant therapy should be stopped before the vast majority of surgical and other invasive procedures. There are, however, cases in which maintaining treatment carries a very low risk of bleeding that may be acceptable to the operator.35 The BRUISE CONTROL trial36 showed that maintaining VKA therapy (INR < 3) in patients with a high risk of thromboembolism who required pacemaker or defibrillator surgery was associated with a lower risk of bleeding than bridging therapy with low-molecular-weight heparin (LMWH).34 The recent results of the BRUISE CONTROL-2 trial showed no differences in the incidence of ischemic and/or bleeding events between patients who stopped and who continued taking direct oral anticoagulants (DOACs).37 In the COMPARE trial of patients with pulmonary vein catheter ablation, those who discontinued anticoagulant therapy with an oral VKA (IRN 2-3) had a lower risk of bleeding and thromboembolic events than those who switched to LMWH.38 Although recent evidence suggests that certain procedures are safe when DOAC therapy is not withdrawn, more studies are needed to allow a definitive recommendation.39 In conclusion, this working group recommends considering not stopping anticoagulant therapy for low-risk procedures with a risk of very minor or clinically insignificant bleeding deemed acceptable by the operator (Table 1 of the supplementary material).
When Should Anticoagulant Therapy Be Stopped?The moment at which anticoagulant therapy should be stopped is largely determined by the drug's elimination route. VKAs are metabolized mainly through the liver and only minimally through the kidneys. With the exception of patients with a liver disease that alters drug behavior (and in whom anticoagulants are generally contraindicated), we recommend stopping acenocoumarol 3 days before an intervention and warfarin 5 days before (Figure 2).9,16 This recommendation, however, is only applicable to patients with INR values within the therapeutic range (INR 2-3) 7 days before the intervention. In patients with supratherapeutic INR values or scheduled for a procedure with a high risk of bleeding, the moment for withdrawing anticoagulant therapy will depend on the exact figure and on the target figure for the operation or procedure. In patients with subtherapeutic INR values, VKAs can be discontinued 1 day later (2 days before the procedure for acenocoumarol and 4 days for warfarin). INR values should always be checked before the procedure. In general, the target should be an INR < 1.5. As DOACs have predictable pharmacokinetics and depend on renal function, the decision on when to withdraw them will depend on the creatinine clearance rate and the risk of bleeding associated with the scheduled procedure.34,40,41
Is Bridging Therapy Necessary?In general, there is a low risk of thromboembolism when oral anticoagulant therapy is discontinued without bridging therapy with heparin.42 In addition, recent data suggest that heparin bridging is associated with an increased risk of bleeding but does not confer any benefits in terms of the incidence of thromboembolic events.43 In the BRIDGE trial, which analyzed bridging anticoagulation in 1884 patients with AF,44 no significant differences were observed for rate of thromboembolic events, but patients in the bridging therapy group experienced more bleeding episodes than those in the placebo group. The main limitation of the trial was that it included patients with a low risk of thromboembolism (exclusion of patients with mechanical heart valves, a history of stroke in the previous 3 months, and a mean CHADS2 score of 2.3) and a low risk of bleeding (only 11% of the procedures carried a moderate to high risk). A similar trial is currently underway to assess patients with a high risk of thromboembolism (PERIOP-2, NCT00432796).
Based on the available evidence, we recommend only using heparin bridging for patients with a high thromboembolic risk (Table 2). In patients being treated with a VKA, it is sufficient to start LMWH (or unfractionated heparin in patients with kidney failure and creatinine clearance < 30mL/min) when the INR is less than 2 or, where this value is not available, when 2 to 3 doses of the drug have been omitted. We do not recommend DOACs as bridging therapy (although some protocols still contemplate their use after the omission of 2 to 3 doses of the VKA). The last dose of LMWH should be administered 12hours before the operation or procedure in the case of prophylactic doses and 24hours in the case of therapeutic doses. Unfractionated heparin should be administered 4 to 6hours in advance.
Stratification of Thromboembolic Risk in Patients on Anticoagulant Therapy
| Risk | Indication for anticoagulant therapy | ||
|---|---|---|---|
| Mechanical heart valve | Atrial fibrillation | Venous thromboembolism | |
| High | Mitral valve Tricuspid valve (including biological valve) Aortic valve (monoleaflet) Stroke/TIA in last 6 mo | CHA2DS2-VASc* score 7-9 Stroke/TIA in last 3 mo Rheumatic mitral valve disease | Recent VTE (in last 3 mo) Severe thrombophilia (homozygous factor V Leiden, prothrombin 20210, protein C, protein S, or antithrombin deficiency, multiple defects, antiphospholipid syndrome) |
| Moderate | Aortic valve + 1 risk factor: AF, stroke/TIA > 6 mo ago, DM, HF, age > 75 y | CHA2DS2-VASc 5-6 Stroke/TIA > 3 mo ago | VTE in last 3-12 mo Nonsevere thrombophilia (heterozygous factor V Leiden or prothrombin 20210 A mutation) Recurrent VTE VTE + active cancer |
| Low | Aortic valve without other risk factors | CHA2DS2-VASc 1-4 No history of stroke/TIA | VTE > 12 mo previously |
AF, atrial fibrillation; DM, diabetes mellitus; HF, heart failure; TIA, transient ischemic attack; VTE, venous thromboembolism.
Achieving adequate hemostasis during the procedure and absence of postprocedural bleeding are the 2 most important factors to consider when restarting a patient on anticoagulant therapy. The moment at which the drug is to be reintroduced should therefore be decided in conjunction with the surgical/intervention team. As a general rule, and under the previous proviso, we recommend resuming anticoagulant therapy 24hours after the procedure. As the anticoagulant effect of VKAs starts to set in at 24 to 72hours, we recommend using heparin bridging therapy in patients with a high thromboembolic risk. This is not necessary with DOACs.41 Reintroduction of oral anticoagulant therapy should be postponed for 48 to 72hours only in patients with a high risk of postoperative bleeding (Figure 2). If it is not possible to restart oral anticoagulant therapy, we recommend evaluating the option of parenteral anticoagulation, preferably with balanced doses of LMWH to cover the risk of thrombosis and minimize that of bleeding.
RECOMMENDATIONS FOR STOPPING AND RESTARTING ANTIPLATELET THERAPYDecisions regarding antiplatelet strategies during the perioperative/periprocedural period depend not only on the balance between thrombotic and bleeding risks but also on the type of antiplatelet therapy and the corresponding indication.5,9,10 It is important to note that antiplatelet therapy is currently not recommended for primary prevention.7 A simple algorithm based on a series of questions designed to facilitate decisions on antiplatelet strategies is presented in Figure 3.
Recommendations for stopping and restarting antiplatelet therapy according to type of intervention and thrombotic and bleeding risk. DAPT, dual antiplatelet therapy. bClassification of thrombotic risk (Table 3). bClassification of hemorrhagic risk (Table 1 of the supplementary material). cIf necessary, withdraw aspirin 3 days before the intervention.
Aspirin should be maintained in patients on single antiplatelet therapy, as it has been found to reduce ischemic risk without significantly increasing bleeding risk.45 Withdrawal for 3 days should only be considered for procedures with a very high risk of bleeding (eg, neurosurgical procedures).9 Unless contraindicated, a low dose (100mg/d) should be administered and maintained, although detection of higher doses (eg, 300mg) before the procedure does not justify deferral. In patients on P2Y12 inhibitor monotherapy, we recommend withdrawing the drug 3 to 7 days before the procedure (3-5 days for ticagrelor, 5 days for clopidogrel, and 7 days for prasugrel) and replacing it with aspirin100mg where possible.45
In patients receiving DAPT and scheduled for an elective procedure, the first step should be to consider postponing the procedure if the patient has a moderate-to-high risk of thrombosis. Where possible, the procedure should be deferred until the risk is low. In other cases, we recommend the following:
- •
Maintain aspirin for all procedures unless contraindicated (eg, neurosurgery).
- •
In patients with a moderate risk of thrombosis (and with the exception of procedures with a low bleeding risk), discontinue the P2Y12 inhibitor 3 to 7 days before the procedure as follows: 3 to 5 days for ticagrelor, 5 days for clopidogrel, and 7 days for prasugrel.5,9 For emergency procedures that can be deferred for 72hours (eg, hip fracture surgery), evaluate the indication for surgery 3 days after withdrawing ticagrelor or clopidogrel and 5 days after withdrawing prasugrel.5,9,30
- •
The strategy in patients with a high thrombotic risk will depend on the bleeding risk associated with the procedure. If this is low, we recommend not stopping DAPT. Cases of moderate-to-severe risk are more controversial and we therefore recommend evaluating patients on a case-by-case basis within a multidisciplinary team. In general, it is important not to stop DAPT in the 30 days after the index event for which it was prescribed.5
Unlike the case of anticoagulation, there are few publications and little clinical experience with the use of bridging therapy in patients on antiplatelet therapy. It is a common mistake to consider heparin in this setting, as heparin can increase platelet reactivity and have the opposite effect to that desired.13 If bridging therapy is necessary, we recommend using an antiplatelet rather than an anticoagulant, as heparin can be harmful.12 Of note among the intravenous antiplatelet agents studied to date are the glycoprotein IIb/IIIa inhibitors, tirofiban and eptifibatide, and the P2Y12 adenosine diphosphate receptor inhibitor, cangrelor.
The perioperative use of intravenous tirofiban and eptifibatide, 2 fast-acting glycoprotein IIb/IIIa inhibitors, has been evaluated in small series of patients with a high thrombotic risk (mainly patients treated with PCI and DES placement), and the results have been controversial.46 Cangrelor, an intravenous reversible P2Y12 inhibitor, has been evaluated in a phase II trial of patients on DAPT who underwent coronary artery bypass grafting surgery.47 Compared with placebo, the drug was associated with a similar incidence of thrombotic events and a slightly higher number of minor bleeding episodes. The trial, however, was designed to study platelet reactivity and not thrombotic or bleeding events, and therefore the findings should be interpreted with caution. Furthermore, the perioperative use of cangrelor has not yet been approved. For the moment, the drug is only indicated for PCIs.
Accordingly, and considering the little evidence available for bridging therapy in patients on antiplatelet therapy, we recommend restricting its use to patients with a high thrombotic risk (essentially patients receiving DAPT for an event that occurred in the previous 30 days) who are scheduled for an unpostponable operation or procedure with a moderate to severe risk of bleeding. The risk-benefit relationship in such cases should be analyzed on a case-by-case basis by a multidisciplinary team. If it is decided that bridging therapy is indicated, the patient should be started on a glycoprotein IIb/IIIa inhibitor 72hours after withdrawal of the P2Y12 inhibitor. The recommended doses are 0.1μg/kg/min for tirofiban and 2μg/kg/min for eptifibatide and these should be maintained for up to 4 to 6hours before the procedure. A loading dose is not required.
Restarting Antiplatelet Therapy: When and How?The 2 most important considerations when restarting a patient on antiplatelet therapy are having achieved adequate hemostasis during the procedure and absence of postprocedural bleeding. The moment at which the drug is to be reintroduced should thus be decided in conjunction with the surgical/intervention team. As a general rule, and under this proviso, we recommend resuming antiplatelet therapy 24hours after the procedure. If the patient has a high risk of thrombosis and is receiving DAPT, consider restarting the P2Y12 inhibitor with a loading dose of 300 to 600mg for clopidogrel, 60 mg for prasugrel, and 180 mg for ticagrelor. Restarting of oral anticoagulant therapy should only be delayed (for 48 to 72hours) in patients with a high risk of postoperative bleeding (Figure 3).
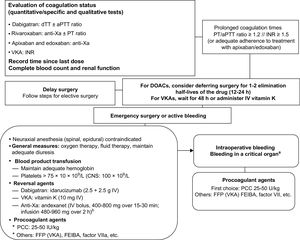
ANTICOAGULATION AND EMERGENCY SURGERYPatients in need of emergency surgery or an emergency invasive procedure do not have the luxury of time associated with an elective procedure. In such cases, it is important to determine the following aspects as quickly as possible (Figure 4)40,48:
- •
The coagulation status of the patient via laboratory tests (INR for patients on VKA therapy). Quantitative tests offer greater precision in the case of DOACs, but as these are often not available, routine qualitative tests, such as activated partial thromboplastin time for dabigatran and prothrombin time for rivaroxaban and probably also edoxaban, can be useful. In most cases, a normal result (understood as a value < 1.2) would strongly rule out significant anticoagulant activity, indicating that the intervention can proceed as planned.49 The hemostasis laboratory should be familiar with the sensitivity of the different coagulation tests to these drugs.
- •
A patient on VKA therapy with an INR < 1.5 can undergo surgery without delay. If the value is higher, the decision will depend on the urgency of the situation. If the procedure can be deferred for 8 to 12hours, the patient should be administered 10mg of intravenous vitamin K. When emergency surgery is required, the most widely accepted option is to administer prothrombin complex concentrate adjusted to the patient's weight (25-50 IU/kg depending on the current and target INR).50
- •
In the case of DOACs, if the hemostasis assay shows anticoagulant activity (or if adherence to treatment with apixaban or edoxaban is adequate), the patient's clinical situation (bleeding, hemodynamic stability, presence of a life-threatening situation, or risk of sequelae) should be evaluated to determine the maximum time for which surgery can be deferred. Ideally, it should be delayed for at least 24hours from the last intake of DOAC (ie, for at least 2 elimination half-lives of the drug in patients with normal renal function).17 In other cases, the option of using a reversal agent should be evaluated in accordance with the type of procedure, the time since the last drug intake, and the patient's renal function. As a general rule, neuraxial anesthesia should be avoided in these patients.40
Recommendations for treating patients on anticoagulant therapy who require emergency surgery. aPPT, activated partial thromboplastin time; CNS, central nervous system; dTT, diluted thrombin time; DOAC, direct oral anticoagulant; factor VIIa, activated factor VII; FFP, fresh frozen plasma; INR, international normalized ratio; IV, intravenous; PCC, prothrombin complex concentrate; PT, prothrombin time; VKA, vitamin K antagonist; Xa, factor X activated. aCNS, pericardial, intraocular, intra-articular, or muscular with compartment syndrome. bAndexanet is not currently authorized for clinical use in Spain (dose as per summary of product characteristics).
Reversal agents act as antidotes by inhibiting the effects of anticoagulants.51 Patients on VKA therapy should be administered intravenous vitamin K (5-10mg depending on INR). However, as vitamin K does not take effect for 6 to 8hours, this strategy will be of little use in patients who require emergency surgery or have active bleeding. In such cases, the option of administering a hemostatic agent should be evaluated.48 Idarucizumab is a humanized monoclonal antibody fragment that completely and selectively inhibits the anticoagulant activity of dabigatran in a question of minutes. It has been shown to be very effective in clinical practice studies.52 It is administered by rapid intravenous infusion (two 2.5-g vials administered 15minutes apart) and does not require complex coagulation assays. Idarucizumab is indicated for patients under the anticoagulant effect of dabigatran who have major acute bleeding or bleeding in a critical organ and/or who require emergency surgery or an emergency invasive procedure with a high risk of bleeding in the next 8hours.53 New reversal agents currently under development are the specific factor Xa inhibitor andexanet af*lfa (intravenous bolus dose of 400-800mg administered over 15-30minutes followed by an infusion dose of 480-960mg administered over 2hours) and ciraparantag, a reversal agent with a broader spectrum of action.53–55
Procoagulant agents are products that provide procoagulant factors and other substances to the coagulation cascade and contribute to the generation of thrombin and hence the achievement of effective hemostasis. They are not reversal agents.53 A human plasma-derived prothrombin complex concentrate containing factors II, VII, IX, and X is the hemostatic agent of choice, although, depending on the dose, the need for repeat use, and the underlying disease, there may be a risk of thrombotic complications. Unlike the case with VKAs, there is little very experience with the use of prothrombin complex concentrates in patients taking DOACs.
Fresh frozen plasma is a second-choice hemostatic agent and is only effective (at a dose of 15-30mL/kg) in patients receiving VKA therapy. It should only be used when no other hemostatic agents are available. Finally, there is very little experience with other hemostatic agents, such as FEIBA, an activated prothrombin complex concentrate that is indicated exclusively for use in patients with hemophilia with inhibitors, or activated recombinant factor VII, which has no established indication in this field and in addition carries a high risk of thromboembolic complications.48,53
ANTIPLATELET THERAPY AND EMERGENCY SURGERYGenerally speaking, treatment with an antiplatelet agent before an emergency operation or procedure is of little consequence, even in elderly patients scheduled for neurosurgery.10,56 It is widely agreed that, following an evaluation of the risk of bleeding versus the need for the procedure, deferral of surgery because of antiplatelet therapy is not justified (even in patients on DAPT).57
Some clinical guidelines suggest considering platelet function tests in selected cases as detection of normalization would shorten the antiplatelet withdrawal time. The use of these tests, however, is limited as they are not usually available in emergency laboratories.5
From both a practical perspective and to guide the choice of anesthesia, it is useful to take note of the antiplatelet the patient is taking. Neuraxial anesthesia, for example, is acceptable in patients being treated with aspirin, but is not recommended in patients undergoing active treatment with a P2Y12inhibitor.9,30 It is also important to emphasize that the most useful and proven strategies for treating a major bleeding episode that is not caused by another coagulation disorder or simultaneous anticoagulant therapy are achievement of surgical hemostasis and intraoperative platelet transfusion. Prophylactic platelet transfusion is not indicated, and, to be effective, would need to be performed at least 6 to 12hours between drug intake and transfusion.9,30 There is much less consensus on the role of hemostatic agents in patients on antiplatelet therapy. Desmopressin has been found to be useful in different heart surgery studies, although results vary considerably from one study to the next.58 There is thus insufficient evidence to justify the systematic use of hemostatic agents and less still the administration of hemostatic agents such as prothrombin complex concentrate, FEIBA, and activated factor VII that generate thrombin and therefore act at a different level of the coagulation cascade to antiplatelets and carry a high prothrombotic risk. This is a particularly important consideration in patients with stable ischemic disease.9,30
CONCLUSIONSVariations in antithrombotic management strategies during the perioperative or periprocedural period are a common problem in everyday clinical practice. The aim of this consensus document, which draws on the opinions of experts across Spanish scientific societies involved in perioperative care, is to synthesize the most important findings on how to manage anticoagulant and antiplatelet drugs and present our recommendations in a simple, practical, and easy-to-apply format. It is essential to create local multidisciplinary working groups to evaluate the available evidence and recommendations, such as those provided in this document, so that they can be adapted to local needs and resources and help to standardize care.
CONFLICTS OF INTERESTNone declared.