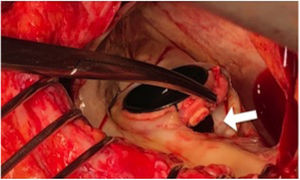
Paravalvular leaks (PVLs) are abnormal communications between the valvular prosthesis sewing ring and the surrounding cardiac tissue (figure 1). Although most PVLs are clinically insignificant, it is estimated that between 2% and 5% cause congestive heart failure, hemolytic anemia, or both.1
Traditionally, surgery has been the only corrective treatment for PVLs. Indeed, it is still considered the technique of choice because it improves survival and reduces symptoms in patients with significant PVL vs conservative treatment.2 Surgical options include PVL repair or prosthesis replacement. The type of surgery depends on the size and extent of the PVL, the condition of the native valve ring, and the patient's surgical history. Nonetheless, regardless of the technique used, reoperation due to PVL is associated with a higher risk of mortality and morbidity than the first surgery, particularly for mitral valve interventions. In addition, after repeated operations, there is a considerable risk of PVL recurrence due to persistent calcification or underlying tissue friability.3
Since Hourihan first described the procedure in 1992, interest has continually grown in the use of transcatheter techniques to treat PVL.4 These approaches were initially reserved as an alternative to medical treatment for inoperable patients or patients with high surgical risk but many experienced centers have recently adopted percutaneous techniques as first-line therapy for patients with PVL.5
Until a few years ago, the global scientific evidence regarding these techniques was limited to single-center studies without long-term clinical follow-up. However, the national registries of Spain6 and the United Kingdom and Ireland7 have recently been published, as well as several studies comparing the outcomes of surgical treatment and transcatheter techniques. Their findings are summarized below.
Millán et al.8 performed a Bayesian meta-analysis including 12 studies and 362 patients with PVL treated with transcatheter techniques. Most of the procedures (70%) were performed on mitral PVL. Procedural success—defined as release of the closure device without prosthesis interference and a reduction of at least 1 grade in the regurgitation severity—was observed in 76.5% of patients, with a slightly lower success rate for mitral procedures than for aortic procedures (73.3% vs 84.1%).
Compared with the failed procedures, successful percutaneous management of PVLs was associated with lower cardiac mortality (odds ratio [OR]=0.08; 95% credibility interval [95%CrI], 0.01-0.90), a greater improvement in New York Heart Association (NYHA) functional class or hemolysis (OR=9.95; 95%CrI, 2.1-66.7), and less need for reoperation (OR=0.08; 95%CrI, 0.01-0.40).
SURGERY VS TRANSCATHETER TECHNIQUESNo randomized studies have compared surgery with transcatheter techniques in terms of PVL management. The evidence is thus limited to retrospective studies, shown in table 1. Taramasso et al.,9 who were the first to compare the 2 therapeutic strategies for mitral PVL, found that percutaneous management was associated with lower 30-day mortality than conventional surgery (0% vs 9.3%) but maintained a high procedural success rate (94%). However, the less invasive procedures in their work were not exclusively transcatheter because all of them were performed through a transapical approach, after an anterolateral minithoracotomy.
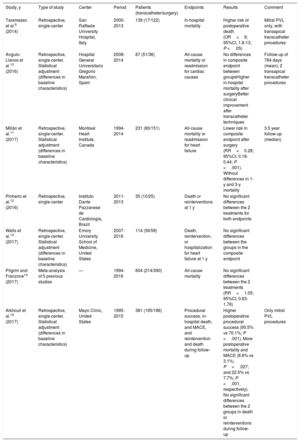
Studies comparing surgical and percutaneous management of PVL
| Study, y | Type of study | Center | Period | Patients (transcatheter/surgery) | Endpoints | Results | Comment |
|---|---|---|---|---|---|---|---|
| Taramasso et al.9 (2014) | Retrospective, single-center | San Raffaele University Hospital, Italy | 2000-2013 | 139 (17/122) | In-hospital mortality | Higher risk of postoperative death (OR=8; 95%CI, 1.8-13; P <.05) | Mitral PVL only, with transapical transcatheter procedures |
| Angulo-Llanos et al.10 (2016) | Retrospective, single-center. Statistical adjustment (differences in baseline characteristics) | Hospital General Universitario Gregorio Marañón, Spain | 2008-2014 | 87 (51/36) | All-cause mortality or readmission for cardiac causes | No differences in composite endpoint between groupsHigher in-hospital mortality after surgeryBetter clinical improvement after transcatheter techniques | Follow-up of 784 days (mean); 2 transapical transcatheter procedures |
| Millán et al.11 (2017) | Retrospective, single-center. Statistical adjustment (differences in baseline characteristics) | Montreal Heart Institute, Canada | 1994-2014 | 231 (80/151) | All-cause mortality or readmission for heart failure | Lower risk in composite endpoint after surgery (RR=0.28; 95%CI, 0.18-0.44; P <.001). Without differences in 1-y and 3-y mortality | 3.5 year follow-up (median) |
| Pinheiro et al.12 (2016) | Retrospective, single-center | Instituto Dante Pazzanese de Cardiología, Brazil | 2011-2013 | 35 (10/25) | Death or reinterventions at 1 y | No significant differences between the 2 treatments for both endpoints | |
| Wells et al.13 (2017) | Retrospective, single-center. Statistical adjustment (differences in baseline characteristics) | Emory University School of Medicine, United States | 2007-2016 | 114 (56/58) | Death, reintervention, or hospitalization for heart failure at 1 y | No significant differences between the groups in the composite endpoint | |
| Pilgrim and Franzone14 (2017) | Meta-analysis of 5 previous studies | — | 1994-2016 | 604 (214/390) | All-cause mortality | No significant differences between the 2 treatments (RR=1.05; 95%CI, 0.63-1.76) | |
| Alkhouli et al.15 (2017) | Retrospective, single-center. Statistical adjustment (differences in baseline characteristics) | Mayo Clinic, United States | 1995-2015 | 381 (195/186) | Procedural success, in-hospital death, and MACE, and reintervention and death during follow-up | Higher postoperative procedural success (95.5% vs 70.1%; P <.001). More postoperative mortality and MACE (8.6% vs 3.1%; P=.027; and 22.5% vs 7.7%; P <.001, respectively). No significant differences between the 2 groups in death or reinterventions during follow-up | Only mitral PVL procedures |
95%CI, 95% confidence interval; MACE, major adverse cardiovascular events; OR, odds ratio; PVL, paravalvular leak; Ref, reference; RR, relative risk.
Adapted with permission from Giblett et al.16
The study by Angulo-Llanos et al.10 compared outcomes between the complete percutaneous management of 51 patients and the surgical treatment of 36 patients. They concluded that in-hospital mortality was lower in the transcatheter group than in the surgical group (9.8% vs 30.6%; P=.002). In addition, the clinical improvement after 2 years of follow-up was better (71.4% vs 36.4%; P=.002). There were no differences between the 2 groups in the composite endpoint of death or hospitalization for heart failure.
In the study by Millán et al.,11 the results of 151 patients who underwent surgery were compared with those of 80 patients who underwent a transcatheter procedure. Despite not reaching statistically significant differences, the transcatheter group had lower perioperative mortality (2.5% vs 6.6%) and no serious complications (0% vs 2.3% for myocardial infarctions and 0% vs 4.6% for strokes). In terms of long-term outcomes, with a median follow-up of 3.5 years, surgical treatment was associated with a 72% reduction in the composite endpoint of death or hospitalization for heart failure (95% confidence interval [95%CI], 0.18-0.44; P <.001). However, in terms of all-cause mortality and without considering hospitalizations for heart failure, no differences were documented between the 2 therapeutic strategies at 1 year or 3 years of follow-up.
Similarly, in a small series of 35 patients, Pinheiro et al.12 failed to find significant differences in mortality or rehospitalization at 1 year of follow-up. The authors attribute the observed tendency toward higher mortality after transcatheter treatment (20% vs 0%; P=.08) to the higher comorbidity of this group of patients.
More recently, Wells et al.13 studied 114 patients with PVL (56 treated percutaneously and 58 surgically). The primary endpoint was a composite of death, reintervention, and readmission for heart failure. After statistical adjustment due to the baseline differences between the groups (higher comorbidity in the percutaneous group), the authors concluded that percutaneous management had equivalent clinical outcomes to surgical treatment at 1 year of follow-up but lower perioperative morbidity.
In addition, the results are available of a meta-analysis of the 5 above-mentioned studies that compared surgical and transcatheter strategies for PVL management.14 With a total of 604 patients included, no significant differences were observed in terms of all-cause mortality between the 2 therapies (relative risk=1.05; 95%CI, 0.63-1.76). The authors admit that the accumulated evidence remains inconclusive and that their results highlight the advantages and disadvantages of the 2 strategies. Due to the heterogeneity in the studies included in terms of PVL location (mitral in 61%-80% of patients) and the transcatheter techniques used (purely percutaneous or via a transapical approach), interpretation of the results is complex.
Finally, the Mayo Clinic15 reported their results after a comparison of the 2 therapeutic strategies for mitral PVL between 1995 and 2015; it is the largest series published to date, with a total of 381 patients (195 treated percutaneously and 186 surgically). As in the other studies presented, surgical management achieved better technical success rates than transcatheter procedures (total or almost total resolution of the PVL: 95.5% vs 70.1%; P <.001) but was associated with worse perioperative morbidity and mortality (8.6% vs 3.1%; P=.027). After adjustment for previous comorbidities, there were no significant differences between the 2 therapeutic modalities in terms of mortality or need for reintervention during follow-up.
The current tendency of many centers to consider transcatheter techniques the first-line therapy for patients with PVL is based on the considerations set forth below. The first point is the low rate of complications during these less invasive interventions, even in the case of unsuccessful procedures or those with incomplete resolution of the PVL. Indeed, in the Spanish HOLE registry (which included 514 procedures in 469 patients from 19 centers), 80.2% of the patients did not experience any complications and the most frequent one was minor bleeding related to the vascular access (8.6%), generally without clinical impact.6 The 30-day incidence of major complications (death, stroke, or need for emergency surgery) was 5.6%. Similar results can be extracted from the registry of the United Kingdom and Ireland (259 patients from 20 different centers), which showed a hospital mortality rate of 2.9% for elective procedures.7 The second consideration is the rapid development of transcatheter techniques due to the growing experience of operators. Since the demonstration of a learning curve, the above-mentioned studies all highlight the relationship between operator experience and favorable results after percutaneous management of PVLs.17 The Spanish registry showed a higher success rate for mitral procedures in centers with more experience.6 The emergence of new devices specifically designed for PVL management (similar to the Occlutech Paravalvular Leak Device, the widely used Amplatzer Vascular Plug III has also obtained the CE mark for use in PVL) and the possible fusion of different imaging modalities (computed tomography or intraprocedural transesophageal echocardiography) with fluoroscopy make transcatheter PVL treatment an increasingly effective and safe procedure.18 Finally, because transcatheter procedures do not prevent or limit future surgical interventions, these techniques should be considered as the initial treatment for patients with symptomatic PVL.
However, it should be noted that the surgical management of PVLs obtained better long-term clinical benefits than transcatheter techniques in some of the studies presented. There may be different reasons for these results: on the one hand, the determining role of the outcome of the intervention. In this regard, the success rates of surgery (>95%) exceed those of transcatheter techniques (70%-90%). In addition, most of the successful transcatheter procedures fail to completely resolve the PVL, which leads to a higher risk of hospitalizations for heart failure and a worse functional class during follow-up but does not necessarily result in higher mortality. In fact, the successful treatment of PVL through transcatheter techniques is associated with the same risk of death at 3 years of follow-up as that of surgically managed patients.11
In addition, the advantages observed after surgery with respect to percutaneous management of PVL should be interpreted with caution due to the differences between the 2 groups of patients. Despite the various statistical adjustment strategies used in the different studies, patients undergoing transcatheter therapy were significantly older and had more comorbidities than patients undergoing surgery, which probably led to their worse clinical course.
In summary, in the absence of randomized studies, the accumulated evidence shows that transcatheter techniques are an effective and safe option for high-risk patients, such as those with valvular prostheses with symptomatic PVL, and compare positively with surgical treatment. Thus, we believe that this approach should not be limited to critically-ill patients rejected for surgery but should be considered the therapeutic option of choice in experienced centers.
CONFLICTS OF INTERESTD. Arzamendi has received fees from Abbott Laboratories, unrelated to the current work. The other authors do not declare conflicts of interest.
.