Paroxysmal supraventricular tachycardias are fast and usually regular rhythms that require some structure above the bifurcation of the His bundle to be continued. The 3 most common types are atrial tachycardias, atrioventricular nodal reentrant tachycardias, and tachycardias mediated by an accessory pathway. The last two varieties are discussed in the present manuscript. Their prognosis is benign regarding life expectancy but typically they are symptomatic and chronically recurrent, producing a certain disability. They usually occur in people without structural heart disease. Pharmacologic therapy is possible, but given the high efficacy of catheter ablation, these procedures are frequently chosen. Ventricular preexcitation is due to the presence of an accessory pathway, usually atrioventricular. The clinical course can be asymptomatic, generating a characteristic electrocardiographic pattern, produce paroxysmal supraventricular tachycardias, or facilitate other types of arrhythmias. Very rarely, they can cause sudden cardiac death. The treatment of choice for symptomatic patients is catheter ablation of the accessory pathway. The therapeutic attitude towards asymptomatic preexcitation remains controversial.
Keywords
.
Concept and classificationSupraventricular tachyarrhythmias (SVT) are rapid rhythms that require a structure above the bifurcation of the bundle of His in order to be sustained. This definition requires some qualification: a) it involves the intuitive concept of a top-down distribution of the heart anatomy, in which the atria are above the atrioventricular (AV) system (AV node, bundle of His, branches, Purkinje network) and the ventricles below, which does not entirely match anatomical reality; b) it is a mechanistic concept, not electrocardiographic, and c) this concept contrasts with ventricular tachyarrhythmias, which do not require a structure above the bifurcation of the bundle of His in order to be sustained, but does not exclude the possibility that the ventricle may be involved (in addition to supraventricular structures) for the existence of the tachycardia, which occurs in some types of SVT.
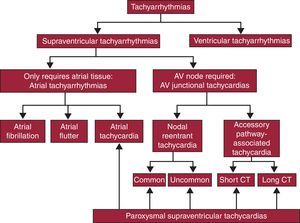
In some SVTs the tissue needed to sustain the arrhythmia is exclusively located in the atrium (atrial tachyarrhythmias), whereas other SVTs require AV junctional structures, usually part or all of the AV node itself, in order to be sustained (AV junctional tachycardias). In general, the term tachycardia refers to tachyarrhythmias with a regular rhythm, at least at the atrial level, in contrast to irregular atrial arrhythmias, typically atrial fibrillation. A particular type of tachycardia has been specifically named atrial flutter. Atrioventricular junctional tachycardia is subdivided into 2 types: those in which the only AV junction structure required is the AV node itself (nodal reentrant tachycardia) and those in which an accessory pathway connecting the atria and ventricles, apart from the AV node, participates as a necessary part of a reentrant mechanism (accessory pathway tachycardias). This classification is presented in Figure 1. The 2 most common types of AV junctional tachycardia are shown in Figure 2.
Figure 1. Proposed classification of tachyarrhythmias. Arrhythmias that are included in the concept of supraventricular tachycardias are highlighted (bottom). AV, atrioventricular; CT, conduction time.
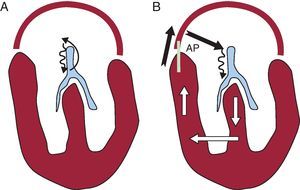
Figure 2. Diagram illustrating the two most common types of paroxysmal supraventricular tachycardia. Anatomical system: the atrial and ventricular architecture is shown in red and the atrioventricular conduction system in blue in both panels. A, Nodal reentrant tachycardia; this is a reentry mechanism involving all or part of the atrioventricular node as the only atrioventricular junctional structure needed (thin arrows in black). B, Accessory pathway-mediated tachycardia; the reentry mechanism involves the atrioventricular node, the His-Purkinje system, ventricular tissue, and the accessory pathway itself through which conduction returns to the atrium and which is then used for new conduction back to the atrioventricular node. AP, accessory pathway.
The clinical presentation of atrial and AV junctional tachycardias is usually abrupt, with episodes that begin and end suddenly (paroxysms), and for this reason are known as paroxysmal tachycardias. Therefore, the term paroxysmal supraventricular tachycardia, which gives this article its title, refers to regularity (tachycardia), sudden onset (paroxysmal), and the mechanism (supraventricular), and encompasses reentrant atrial tachycardias, nodal reentrant tachycardias, and those involving accessory pathways (Figure 1). As tachycardia and atrial flutter are addressed in another article in the present series named “Update: Arrhythmias”, we refer exclusively to paroxysmal tachycardias of the AV junction.
We should mention at the outset that the best tool for diagnosing SVT is the electrocardiogram (ECG) and the 2 major electrocardiographic markers of these tachycardias are narrow QRS complexes and regular RR intervals. The former are due to the fact that ventricular activation occurs through the specific conduction system, as well as during sinus rhythm (Figure 2), and the latter are due to the regularity mentioned above to which the term tachycardia alludes. However, although these 2 markers are always present in SVT, in some cases the QRS complex may be wide or the intervals irregular in these tachycardias.
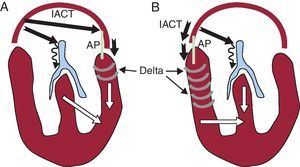
Closely related to SVT are the ventricular preexcitation syndromes, which have received this name because the ventricles begin their electrical activation (excitation) sooner than usual, hence the term “preexcitation” or Wolff-Parkinson-White syndrome, which refers to the researchers who described it. This phenomenon is due to an abnormal connection or “accessory pathway” that typically “jumps” across the mitral or tricuspid valve annuli, proximally inserted in an atrial site and distally in a ventricular site. Given that conduction in these accessory pathways tends to occur faster than in the AV node, the impulses coming from the atria reach a part of the ventricles sooner than would be expected if the spread of the impulses from the atria had passed exclusively through the specific conduction system, ie, the ventricles (at least part of them) are preexcited (Figure 3). As the accessory pathway involves the same substrate that causes “accessory pathway tachycardias”, it is common for the patient with preexcitation syndrome to also have SVT. Due to this close relationship, this article also addresses preexcitation syndromes.
Figure 3. Diagram illustrating ventricular preexcitation during sinus rhythm. The anatomical system is the same as in Figure 2 . Both panels show ventricular preexcitation situations depending on whether the accessory pathway is left-sided (A) or right-sided (B). The thick black arrows indicate the intraatrial conduction time from the sinus node to the accessory pathway atrial insertion site, the AV node and the conduction time over the accessory pathway. Note that as conduction in the atrioventricular node is slow (thin wavy arrow) whereas conduction in the accessory pathway is rapid, activation through this pathway reaches the ventricles early (preexcitation), indicated by grey crescents (delta wave). Both panels show that ventricular activation partly occurs through the accessory pathway (white arrow) and partly through the specific conduction system (white arrow), leading to a ventricular fusion pattern in the QRS complex. Also note that the amount of ventricular myocardium activated through the accessory pathway (preexcited) is greater in the right pathways than in the left pathways. This is due to the simple fact that the conduction time from the sinus node to the accessory pathway atrial insertion site is shorter. IACT, intra-atrial conduction time; AP, accessory pathway.
For more than 2 decades, invasive studies have been conducted for the diagnosis and characterization of the mechanisms involved in SVT, as well as invasive treatment via catheter ablation of the arrhythmogenic structures. Due to space limitations, this article focuses on clinical and electrocardiographic aspects and therapeutic decision-making, without in-depth discussion of the purely electrophysiological aspects.
Symptoms associated with paroxysmal supraventricular tachycardiasOur starting point is based on evidence that a good clinical approach is the foundation of all diagnostic and therapeutic strategies in SVT. There is a broad and diverse spectrum of symptoms associated with SVT, ranging from patients who are almost asymptomatic to those who present symptoms of low cardiac output and syncope. The degree of tolerance and the severity of the arrhythmia depend on factors such as the type of SVT, heart rate, frequency and duration of the episodes, the presence of structural heart disease, and the patient's subjective perception. These variables taken together will determine what course to follow.
Tachyarrhythmia episodes are often interpreted as anxiety disorders, such that the period from symptom onset to the diagnosis of SVT can be greater than 1 year.1 In this setting, if the patient reports episodes of palpitations that start and end abruptly, with a frequency between 180 bpm and 220 bpm, and that can be interrupted using the Valsalva maneuver, this may be SVT. It should be borne in mind that in many cases SVT episodes are often followed by sinus tachycardia, and thus patients may see a gradual reduction in episodes.
Other important aspects of taking the medical history are to identify possible triggers, age of symptom onset, tolerance to the episode, response to vagal maneuvers or drugs, and a history of cardiac disorder. The SVT episodes often occur in situations of physical or emotional stress, but can also occur at rest. Some patients associate certain movements or postures, such as suddenly bending forward or down, with the onset or termination of the tachycardia. Other predisposing factors are the use of drugs with adrenergic properties, drinking stimulants or caffeine or alcohol (more often associated with the onset of atrial fibrillation).
Although SVTs may occur at any stage of life, they mainly affect young adults. The age of symptom onset in patients with accessory pathway-mediated tachycardia is usually earlier, around 25 years,2 than in patients with nodal reentrant tachycardia, which reaches its highest incidence in the fourth decade of life. There are also sex differences; nodal reentrant tachycardia is more frequent in women and accessory pathway tachycardia is more frequent in men.3
The most common symptom of SVT is regular palpitations. The perception of a pounding sensation in the neck is characteristic of nodal reentrant tachycardias, occurring in up to half of the cases, although it is not specific to this entity. It is due to a simultaneous contraction of the atria and ventricle with the AV valve closed, leading to increased right atrial pressure and venous return. Cannon A waves at the cervical level is known as “frog sign”. This phenomenon is less likely in SVTs in which the AV interval is greater, as occurs in accessory pathway-mediated SVTs, because the pressure gradient is usually lower.4
Polyuria associated with episodes of tachycardia is due to the increased release of atrial natriuretic peptide, which is also a response to increasing pressure at the right atrium, and is typical of patients with nodal reentrant tachycardia.
Dizziness is a common symptom that can reach presyncope or even syncope in 15% of patients. The latter usually coincides with the onset of tachycardia or immediately after its completion, related to the long pauses prior to the restoration of sinus rhythm. Patients are usually older, with rapid SVT, and have underlying structural heart disease (aortic stenosis or hypertrophic cardiomyopathy) or cerebrovascular disease.
The SVT episodes may also involve chest pain, dyspnea or signs of heart failure, especially in the presence of left ventricular dysfunction. Chest pain is often nonspecific and is not indicative of coronary disease. There is a form of SVT which, when continuous, can lead to tachycardia-induced cardiomyopathy, which is wholly or partially reversible after controlling the arrhythmia.
The integration of all the clinical information with the electrocardiographic findings during SVT episodes significantly increases diagnostic accuracy when establishing the arrhythmic mechanism of the SVTs. Our group conducted a prospective study5 of SVT patients who underwent electrophysiological study (n=370). We found that the onset of arrhythmic events at an older age, the perception of beats at the cervical level, and female sex were significantly associated with nodal reentrant tachycardia. The presence of 2 of these variables, with compatible ECG changes, had a diagnostic accuracy for nodal reentrant tachycardia greater than 90%. Even in patients with inconclusive electrocardiographic findings, the presence of palpitations at the cervical level was predictive of nodal reentrant tachycardia.
The response to vagal maneuvers provides relevant clinical information about the possible mechanism of SVT. The effect exerted by the increased parasympathetic tone that temporarily slows AV node conduction is less effective in hemodynamically unstable SVT due to increased sympathetic tone. The best-known technique is the Valsalva maneuver, which causes increased intrathoracic pressure (by the patient trying to exhale air against a closed glottis), stimulates the aortic arch baroreceptors, and activates vagal tone. Massaging the carotid sinus is performed by maintaining a slight pressure at the level of the carotid sinuses for 5-10s. It is contraindicated if the patient has a history of carotid disease or stroke and if murmurs are heard at this level. It is advisable to perform this maneuver during ECG and blood pressure monitoring, due to the possibility of bradycardia and hypotension secondary to the maneuver.
Vagal maneuvers may produce the following responses during SVT: a) slowing atrial rhythm by slowing the sinus node, characteristic of sinus tachycardia; b) slowing or the intermittent blocking of AV nodal conduction, unmasking P waves by reducing the number QRS complexes, characteristic of typical atrial flutter or tachycardia; c) momentary blocking of nodal conduction with interruption of the SVTs which require the AV node in order to be sustained, and d) a lack of response in some cases.
In the era of interventional treatment, the question arises of whether clinical and electrocardiographic information is of any value. The answer is yes if we consider it important to provide patients with accurate information regarding their prognosis and the potential risks of therapeutic electrophysiology study; this allows us to select the tools needed to ensure success.6
Nodal reentrant tachycardia EtiologyNodal reentrant tachycardia is the most frequent SVT, reaching 75% in some series. Other terms used to refer to this arrhythmia include intranodal tachycardia, AV junctional reentrant tachycardia, and reciprocating junctional tachycardia. We have already referred to the fact this disorder is more frequent in women (ratio 2:1).7, 8 Studies have shown that in women the refractory periods of the slow pathway are shorter and the tachycardias are faster, although the anatomical and electrophysiological causes of this difference are still not fully understood.
It mainly occurs in middle-aged individuals, with its peak incidence around 40-50 years, although cases among children and the elderly are not rare. The greater number of patients with nodal reentrant tachycardia in adulthood can be explained by the developmental changes that occur in AV node physiology during the first 2 decades of life. In general, these are patients without structural heart disease, although in older patients the prevalence of heart disease is higher. There are rare cases of familial nodal reentrant tachycardia.
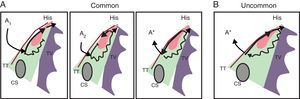
Specific MechanismAlthough the mechanism of this arrhythmia has been well defined in relation to its anatomical and pathophysiological features, various aspects await elucidation. This is a reentrant arrhythmia located in the nodal region within the triangle of Koch (Figure 4). This circuit clearly involves the AV node, with 2 intranodal pathways or connections: a slow conduction pathway with a typically short refractory period and a fast pathway with a typically longer refractory period. There is continuing debate on whether the upper reflection point of the reentry circuit involves a small amount of atrial tissue (in which case it should be called atrionodal tachycardia) or whether the entire circuit is confined to the AV node (in which case it should be called intranodal tachycardia). Therefore, we use the more neutral term “nodal reentrant tachycardia”.
Figure 4. Anatomical and pathophysiological model of nodal reentrant tachycardia. The triangle of Koch is shown bounded by the coronary sinus at the base, the tendon of Todaro, the septal leaflet of the tricuspid valve, and the His region at the apex. The scheme indicates the pathophysiological mechanism of dual nodal pathway and common nodal reentrant tachycardia (A) and uncommon nodal reentrant tachycardia (B). A, During sinus rhythm (A1) the impulse is conducted by the fast pathway to the common terminal region, His, and ventricle, and the impulse that travels along the slow pathway is extinguished; the extrastimulus (A2) reaches the fast pathway in the refractory period and a prolonged PR interval occurs; if the impulse reaches the excitable rapid pathway in a retrograde direction, an echo beat (A*) occurs; this sustained reentry causes the common nodal reentrant tachycardia, also known as slow-fast. B, During uncommon, or fast-slow, nodal reentrant tachycardia, anterograde conduction occurs through the fast pathway with retrograde conduction through the slow pathway. CS, coronary sinus; TT, tendon of Todaro; TV, tricuspid valve.
Common nodal reentrant tachycardia, also called typical or slow-fast AV nodal reentrant tachycardia, is the most common type (between 80% and 90% of cases). During sinus rhythm the impulse travels along both pathways, but arrives early at the bundle of His through the fast pathway. An atrial extrastimulus produced during the time window in which the fast pathway is in a refractory period will be conducted by the slow pathway alone (because it has a shorter refractory period), resulting in a prolonged PR interval. If the excitability of the fast pathway is recovered in the opposite direction while the impulse conducts down the slow pathway, the impulse conducted by the fast pathway will activate the atrium, producing an “echo beat”. If this mechanism is sustained and repeats, conducting down the slow pathway and up the fast pathway, a sustained nodal reentrant tachycardia occurs (Figure 4, Figure 5).
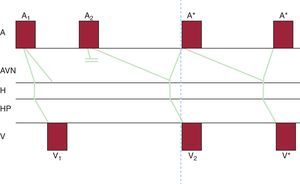
Figure 5. Schematic model of common, or slow-fast, nodal reentrant tachycardia. The phenomena depicted in Figure 4 A are represented on a ladder diagram to better elucidate their temporal relationships. Note that atrial and ventricular activation is virtually simultaneous during tachycardia. A, atrium; A1, sinus impulse; A2, early atrial impulse (extrasystole or extrastimulus); A*, atrial activation during tachycardia; AVN, atrioventricular node; H, bundle of His; HP, His-Purkinje system; V, ventricle; V1, ventricular activation due to a normal sinus impulse; V2, ventricular activation due to an atrial extrasystole conducted by the slow pathway alone; V*, ventricular activation during nodal reentrant tachycardia.
Uncommon nodal reentrant tachycardia, also known as atypical or fast-slow AV reentrant tachycardia, is less frequent (5%-10% of cases). The circuit is sustained in an inverted manner (Figure 4, Figure 6) and during the tachycardia the impulse conducts down the fast pathway and up the slow pathway or reentry occurs along 2 slow pathways (slow-slow).
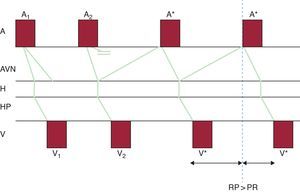
Figure 6. Schematic model of uncommon, or fast-slow, nodal reentrant tachycardia. The phenomena depicted in Figure 4 B are represented on a ladder diagram to better elucidate the temporal relationships between them. Note that atrial and ventricular activation separate resulting in a long RP interval. A, atrium; A1, sinus impulse; A2, early atrial impulse (extrasystole or extrastimulus); A*, atrial activation during tachycardia; AVN, atrioventricular node; H, bundle of His; HP, His-Purkinje system; V, ventricle; V1, ventricular activation due to a normal sinus impulse; V2, ventricular activation due to an atrial extrasystole conducted by the slow pathway alone; V*, ventricular activation during nodal reentrant tachycardia.
The physiology of the dual nodal pathway is shown by programmed atrial stimulation during the electrophysiologic study. By applying stimulation at a specific basic cycle length, atrial extrastimuli are introduced at decrements of 10 ms (A1A2). Initially, impulse conduction takes place through the fast pathway until reaching the refractory period when there is a “gap” ≥50ms in the interval between the local atriogram and His bundle potential (A2H2) when atrial extrastimuli are introduced in decrements of 10 ms (A1A2). The ECG will show a prolonged PR interval at the expense of an increased AH interval.
This dual nodal pathway model fails to explain some issues, such as the fact that in up to 39% of patients with nodal reentrant tachycardia it is not possible to demonstrate dual pathway physiology during the electrophysiological study. On the other hand, up to 30% of patients with a dual intranodal pathway do not have reentrant nodal tachycardia.9 Similarly, there are controversial arguments suggesting an inferior common terminal pathway in the circuit, which would explain the fact that in up to 10% of nodal reentrant tachycardias 2:1 AV conduction can be observed at some point (Figure 7D).
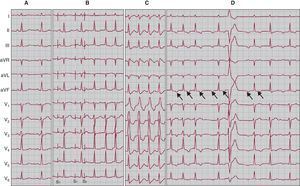
Figure 7. Four electrocardiographic recordings from the same patient. A, 12-lead electrocardiographic during sinus rhythm. B, 12-lead electrocardiographic recording showing the initiation of a common nodal reentrant tachycardia episode; via programmed atrial stimulation with a train of impulses followed by extrastimulus, there is a prolonged PR interval and initiation of tachycardia. C, electrocardiographic recording of the same tachycardia with aberrant right branch. D, Uncommon nodal reentrant tachycardia with a run of second-degree atrioventricular block; arrows indicate the retrograde P wave, negative in leads II, III, and aVF, and isoelectric in lead I.
Electrocardiographic DiagnosisThe electrocardiographic pattern characteristic of nodal reentrant tachycardia is regular, typically with a narrow QRS complex, unless there is aberrant conduction (Figure 7C). The heart rate ranges between 120 bpm and 220 bpm, although occasionally slower tachycardias with a continuous pattern occur.
Common nodal reentrant tachycardia is included within short RP tachycardias. It is not always easy to identify the P wave during tachycardia, since activation of the atria and ventricles occurs almost simultaneously (Figure 5); the retrograde P wave may be hidden within the QRS complex, producing a pseudo-R’ pattern in V1 and a pseudo-S pattern in the inferior leads (Figure 8). To identify these small changes in the QRS complex, it is important to compare the QRS complex morphology during tachycardia to the QRS complex in sinus rhythm, if available (Figure 8). It can also be identified slightly behind the QRS complex, and since atrial activation occurs “bottom up”, the P wave is negative in inferior leads.
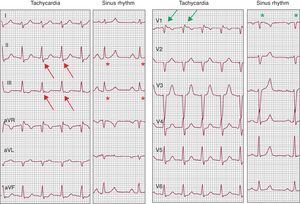
Figure 8. Electrocardiogram during narrow QRS regular tachycardia at 140 bpm compared to sinus rhythm. A nodal reentrant tachycardia is shown in which P waves are recorded at the end of the QRS complex, giving the false appearance of S in inferior leads (red arrows) or of R’ in lead V1 (green arrows). The fact that these deflections do not really form part of the QRS complex is proven by their absence during sinus rhythm (red and green asterisks).
In uncommon nodal reentrant tachycardia, the retrograde P wave appears delayed, slightly ahead of the next QRS complex, and is therefore included within long RP tachycardia. The P wave is negative in inferior leads and isoelectric in lead I (Figure 7D).
Nodal reentrant tachycardia sometimes “deteriorates” into atrial fibrillation, masking the original arrhythmia. In these cases the patient's perception of well-defined regular tachycardia and irregular tachycardia is crucial. The sequence can also be inverse, changing from atrial fibrillation to nodal reentrant tachycardia (Figure 9).
Figure 9. Twelve-lead electrocardiogram during an atrial fibrillation episode (left half of the tracing) that ends by deteriorating into a nodal reentrant tachycardia (right half of the tracing).
Between 25% and 50% of nodal reentrant tachycardias involve a depressed ST segment without underlying coronary artery disease. Once the tachycardia is reversed, there is transient T-wave inversion in up to 40% of cases.
PrognosisThe clinical course of nodal reentrant tachycardia is benign. Serial studies of patients who have not undergone ablation have shown that, over time, the tachycardias remain inducible in 90% of patients, although there is an increase in the refractory periods of the slow and fast pathways and the tachycardias are usually slower.10 These data are consistent with clinical findings that many patients have fewer episodes over time, although the course alternates between more active periods and others with fewer SVT episodes.
TreatmentWe describe acute treatment for terminating tachycardia and long-term treatment.
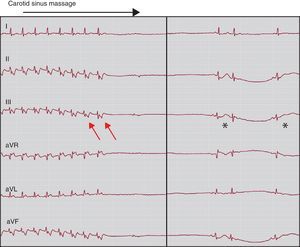
Acute TerminationThe initial treatment depends on the severity of the arrhythmia and level of tolerance. Vagal maneuvers are indicated in patients with well-tolerated SVT,11 and may be useful to terminate reentrant nodal reentrant tachycardias (Figure 10) and those mediated by an accessory pathway (Figure 11).
Figure 10. Aberrant nodal reentrant tachycardia episode interrupted by carotid sinus massage.
Figure 11. An accessory pathway-mediated tachycardia episode interrupted by carotid sinus massage. When the tachycardia is interrupted a prolonged sinus pause occurs. Note the clearly visible P wave during tachycardia, separated from the QRS complex (red arrows). We can be confident that that this is not repolarization due to the observed change when passing to sinus rhythm (asterisks).
If the arrhythmia is poorly tolerated and is associated with signs of hemodynamic deterioration, it should be terminated by the administration of adenosine or by electrical cardioversion. Adenosine is the drug of choice due to its great efficacy and very short half-life (5s). After the administration of a bolus of 6mg or 12mg, the success rate varies between 75% and 90%, depending on the dose administered.12 The limitations of this drug are poor tolerance due to chest pain, vasodilatation, and brief general discomfort. It is contraindicated in asthmatic patients due to the risk of bronchospasm and in patients with coronary disease.
Due to the short half-life of adenosine, in the case of early recurrences, or when adenosine is considered dangerous, drugs with a longer half-life should be used, such as verapamil (5mg to 10mg intravenously [IV]), diltiazem or beta-blockers (metoprolol or esmolol). A dose of verapamil (10mg IV over 5min) has a similar success rate to adenosine, but is contraindicated in patients with hypotension or severe ventricular dysfunction.
Long-term TreatmentThe long-term therapeutic strategy should be individualized, taking into account the frequency and clinical impact of the episode, tolerance of antiarrhythmic drugs, and patient preference.
Some patients who have well-tolerated infrequent episodes that can be terminated using reproducible vagal maneuvers do not need treatment. In other cases, the administration of a single dose of a drug that has been previously assessed under observation is sufficient to terminate an episode without the patient needing to visit the emergency department; this strategy is known as pill-in-the-pocket and avoids the need for chronic drug treatment. However, this strategy takes a long time to achieve its effect (1 h to 3h) and thus is rarely of practical use since the SVT episodes often cause very unpleasant symptoms.
When arrhythmic episodes are frequent and the patient prefers a conservative treatment approach, antiarrhythmic drugs can be used as prophylactic treatment. The most frequently used drugs are verapamil, beta blockers, or class IC antiarrhythmic drugs.
Electrophysiological Study and Catheter AblationAn electrophysiology study for therapeutic purposes in symptomatic patients is indicated when: a) pharmacological control is inadequate; b) there is intolerance to drugs; c) tachycardias are poorly tolerated; d) there is underlying structural heart disease, and e) the patient prefers ablation, particularly in certain circumstances, such as before pregnancy to avoid antiarrhythmic drugs, or in patients whose sporting or professional activities (eg, pilots) require definitive treatment.
The current technique of choice involves modifying the slow pathway. Anatomical and electrophysiological criteria are used to choose the application site. Slow pathway ablation is usually performed in the medial and posterior region of the triangle of Koch, near the coronary sinus ostium, although other structures may be involved in the circuit.13 At this level the atrial-to-ventricular electrographic ratio should be less than 0.5, and ECGs that are fragmented or have characteristic multicomponents, referred to as slow pathway potential, are frequently recorded.14, 15 When radiofrequency energy is used, effective applications usually induce a slow nodal rhythm with ventriculoatrial conduction. If this is accompanied by retrograde block, the application should be immediately terminated. The goal of ablation is to suppress tachycardia inducibility; therefore, it is not necessary to eliminate slow pathway conduction. In fact, the maintenance of dual nodal physiology and the provocation of up to 1 intranodal echo may be an acceptable end-point, without it being associated with a higher recurrence rate.16 The efficacy of slow pathway ablation has been well demonstrated; a registry of different laboratories that treated 8230 patients with nodal reentrant tachycardia achieved 99% efficacy in the long term, as only 1.3% of the patients needed a second procedure, with a rate of iatrogenic AV block that required the implantation of a permanent pacemaker of 0.4%.17 Other registries have reported a success rate of 96% and a risk of AV block of 1%.18 The recurrence rate ranges from 3% to 7% depending on the series.11
Currently, fast pathway ablation is considered an alternative only if slow pathway ablation fails, since it has a lower success rate (80%-90%) and an increased risk of procedure-associated AV block (up to 8%). It is usually associated with prolongation of the PR interval.
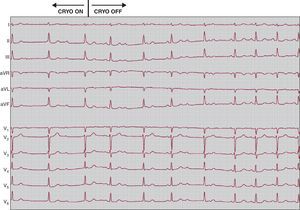
Cryoablation is currently the safest option for catheter ablation of nodal reentrant tachycardia. The greater stability of the catheter, as well as its potentially reversible effect in the case of AV block, can avoid the risk of AV block associated with radiofrequency ablation.19 Although transient AV block during cryoablation has been recorded in up to 6% of cases, it is completely reversible by terminating the application20 (Figure 12). Slow pathway cryoablation has very high efficacy, up to 95%, although the recurrence rate is higher than radiofrequency ablation, ranging between 8% and 14% depending on the series.20, 21, 22 The greater safety of the technique has increased the indications for ablation in pediatric patients.
Figure 12. Twelve-lead electrocardiogram showing a complete atrioventricular block episode with the beginning of cryoablation in the slow pathway site. It can be seen that atrioventricular conduction is normalized a few seconds after the interruption of cryoablation.
Accessory pathways and ventricular preexcitationThe great majority of abnormal connections “jump” the mitral or tricuspid rings, electrically connecting the atria to the ventricles while bypassing the specific conduction system (accessory or abnormal pathways). They consist of normal working myocardial fibers and are caused by abnormal embryologic development of the valvular rings.
Types of Accessory Pathways, Terminology and CharacteristicsThe conduction properties of accessory pathways and, in part, their location determine their possible characteristics:
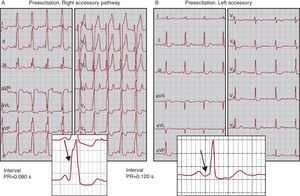
1. If there is anterograde conduction (atrium to ventricle), and given that their conduction time is usually short (conduction velocity in the working myocardium is higher than in the AV node), electrical activation propagates from the atria to the ventricles through the accessory pathway more rapidly than through the specific conduction system and initiates ventricular activation earlier than normal (ventricular preexcitation, see Figure 3); this translates into a short PR interval on ECG (Figure 13A). As the ventricular insertion site of the accessory pathway is usually some distance from the Purkinje network, ventricular activation starts from fiber to fiber, without involving the Purkinje system, and therefore produces slow forces of ventricular activation on ECG, known as the “delta wave” (Figure 3, Figure 13).
Figure 13. Examples of electrocardiograms showing ventricular preexcitation. The arrows indicate the delta wave. Refer to the text for further details.
2. If there is retrograde conduction (ventricle to atrium), under certain conditions this can lead to “reentrant” cardiac activation where the atrial impulses, which propagate to the ventricles via the specific conduction system, return to the atria (“reentrance”), propagating through the accessory pathway. If this mechanism is sustained, there is SVT by reentry or circular movement or involving an accessory pathway. Since in these tachycardias the conduction system is used in the same direction as in normal conditions (atrium to ventricle) they are also called orthodromic tachycardias (Figure 2B).
3. In most cases, accessory pathways have bidirectional conduction, and so may lead to preexcitation patterns on the ECG as well as SVT. This is known as Wolff-Parkinson-White syndrome, although this term is sometimes used to simply refer to ventricular preexcitation.
4. It is relatively common for accessory pathways to only have ventriculoatrial conduction. In these cases, there may be no abnormalities on the ECG in sinus rhythm, but SVT may occur with the participation of accessory pathways (Figure 2B). These types of pathway are known as concealed accessory pathways because they are not seen on the ECG during sinus rhythm.
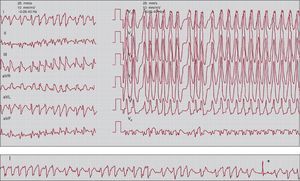
5. Arrhythmias may be associated with ventricular preexcitation. For reasons not fully understood, atrial fibrillation and flutter are more common in people with ventricular preexcitation than in the rest of the population. It is well known that the AV node functions as a physiological filter, such that if the number of atrial impulses it receives is very high, many of them are blocked from propagating through it. This is typically seen during atrial fibrillation or flutter, which limits the ventricular response. In contrast, accessory pathways, which are composed of working myocardial fibers, do not filter the atrial impulses to the same degree (although this function varies from one accessory pathway to another). This may lead to a very high ventricular response rate due to AV conduction predominantly or exclusively through the accessory pathway during atrial fibrillation (Figure 14) or a 1:1 response in the case of atrial flutter (preexcited atrial fibrillation or flutter), which may deteriorate into life-threatening ventricular fibrillation. Regular tachycardias are sometimes observed in which AV conduction occurs via the accessory pathway (preexcited tachycardia), the most characteristic being antidromic tachycardia, an SVT arrhythmia in which AV conduction is through the accessory pathway and retrograde conduction is through the specific conduction system, hence the name. Preexcited tachycardias are rare in clinical practice.
Figure 14. Electrocardiogram showing preexcited atrial fibrillation. Note that the ventricular rate is very rapid and irregular with wide QRS complexes (preexcited) which, in turn, are very similar to each other (differentiating it from polymorphic ventricular tachycardia). The recording strip in the bottom panel shows a narrow QRS complex (asterisk): this is typical of preexcited atrial fibrillation, which is occasionally interspersed with non-preexcited beats.
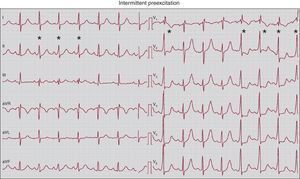
6. Degrees of preexcitation. When the anterograde conduction properties of the accessory pathways are so deficient that some sinus beats propagate through them whereas others do not, the phenomenon called intermittent preexcitation (Figure 15) occurs. On other occasions, there are moments, hours, and days in which ventricular preexcitation appears and others in which it does not appear. In these patients we may see a normal ECG and others with preexcitation. Although ventricular preexcitation exists it can manifest to various degrees on the ECG. Note that the time difference between when the activation front propagated through the accessory pathway arrives at the ventricle and when it propagates through the conduction system depends on the relationship between the atrial, nodal, and accessory pathway conduction times. For example, preexcitation is usually more marked in right-side pathways than in left-sided pathways because the right-sided pathways are closer to the sinus node (Figure 3, Figure 13A,B). Little overt preexcitation on the ECG is known as inapparent preexcitation. It should be recalled that the clinical risk inherent to preexcitation is not related to the degree of preexcitation or the appearance of preexcitation on ECG.
Figure 15. Electrocardiogram showing intermittent ventricular preexcitation. Note that during sinus rhythm a few beats show ventricular preexcitation (marked with an asterisk), whereas some other beats do not show this. In beats with preexcitation, the QRS complex widens and changes in morphology, and the typical features of a delta wave appear (especially clear in V2 to V6) with a shortened PR interval.
7. Atypical accessory pathways. Are those in which proximal insertion is not atrial or distal insertion is not ventricular, both of which are exceptional situations. The only atypical accessory pathways of some relevance are atriofascicular pathways; these are typically right-sided, have long conduction times, and are sometimes known as “Mahaim bundles”. The concept of atrionodal or atrio-Hisian accessory pathways, which were assumed to be associated with tachycardias and short PR without a delta wave in the setting of Lown-Ganong-Levine syndrome, should be abandoned. It is now known that there is no electrophysiological evidence of accessory pathways in these patients; rather, the AV node has shorter conduction times but with normal physiology, and so this syndrome should be discarded given that it is associated with SVT due to any mechanism.
Several clinical observational studies of patients with ventricular preexcitation have been conducted with long follow-up periods.23, 24, 25, 26, 27, 28, 29, 30 The main conclusions are as follows: a) the prevalence of ventricular preexcitation in the general population ranges between 0.1% and 0.4%; b) between 10% and 50% of patients are asymptomatic at the beginning of the follow-up period; c) during long follow-up periods of between 5 and 15 years, a significant minority of patients (up to one third if they are aged less than 40 years at the beginning of the study) will experience supraventricular tachyarrhythmias, typically SVT, although a few will experience atrial fibrillation; d) sudden death is a very rare event, although most studies with a population>100 subjects and long follow-up periods have reported such cases, and e) when sudden death occurs it is characteristically preceded by symptomatic tachyarrhythmias, such that it is virtually nonexistent in previously asymptomatic subjects as the first manifestation of the disease.
Classic studies have established that in patients with ventricular preexcitation who have suffered cardiac arrest, the mechanism was preexcited atrial fibrillation with rapid ventricular response that deteriorated into ventricular fibrillation.31, 32 A necessary condition for this cascade of events is that the accessory pathway allows the conduction of impulses at a high frequency. Numerous studies have focused on noninvasive or invasive parameters associated with the electrophysiological properties of the accessory pathway. It is accepted that intermittent preexcitation33 (Figure 15), block of the accessory pathway during exercise 34 or with procainamide,35 the long effective refractory period of the accessory pathway,36 or the nonrapid ventricular response during induced atrial fibrillation are associated with a “low risk” of sudden death.36, 37 The difficulty with these studies is that they identify a high percentage of patients with ventricular preexcitation as having “high-risk” parameters, yet during clinical follow-up sudden death as the first manifestation of disease is virtually absent. The few patients who died suddenly had previously presented documented spontaneous tachyarrhythmias.37 In other words, the patients with a high-risk profile but without spontaneous arrhythmias are also at low clinical risk of sudden death. A different question is whether we are referring to the risk of experiencing a tachyarrhythmia, as suggested in the title of the study referenced.37 Although this can be predicted by electrophysiological assessment, there is little clinical relevance because SVT, for example, is a benign arrhythmia that can be treated acutely with high efficacy.
The vast majority of patients with accessory pathways do not have associated structural cardiac abnormalities. Ebstein anomaly is the heart disease most frequently associated with preexcitation. Of patients with ventricular preexcitation, 3% have a first-degree relative with preexcitation, suggesting some familial predisposition.
The typical clinical characteristics of SVT have been previously described.
Electrocardiographic AspectsThe diagnosis of preexcitation is based on the recognition of the previously mentioned triad of a short PR interval, a delta wave, and a wide QRS complex (Figure 13). However, the ease or difficulty of recognizing this pattern depends on how well marked it is. For the reasons previously outlined, right or septal pathways typically have more marked patterns and are therefore more easily recognized (Figure 3, Figure 13) than left-sided pathways (Figure 3, Figure 13).
Both the direction of the initial QRS vector (delta wave vector) and the overall morphology of the QRS can assist in identifying the accessory pathway site. Complex algorithms have been described for this purpose38, 39 but they are difficult to remember and have limited sensitivity and specificity for different patterns and sites. Moreover, their practical use is limited; their most useful application might be to detect in advance the septal sites at risk of iatrogenic AV block in the setting of ablation procedures.
As mentioned, the majority of accessory pathway-mediated SVTs are regular narrow QRS tachycardias. On ECG they are quite similar to the nodal reentrant tachycardias referred to previously. One aspect that can contribute to the ECG differential diagnosis is that the P wave in nodal reentrant tachycardia is usually within the QRS complex or at the end (Figure 5, Figure 8), whereas in accessory pathway-mediated SVT the P wave is behind the QRS complex (Figure 11), since the impulse has to travel through part of the ventricle and the accessory pathway before reaching the atrium (Figure 2B). In a narrow QRS tachycardia, if a bundle branch block appears which is associated with a prolonged interval between the QRS complex and the P wave and slowing of the tachycardia, we can be confident that this is due to an accessory pathway ipsilateral to the bundle branch block.
Treatment Acute Termination of Regular Narrow QRS TachycardiaTreatment is the same as that described for nodal reentrant tachycardia. It only remains to add that in the rare event adenosine induces atrial fibrillation and is sustained, if the patient has preexcitation during sinus rhythm the SVT may deteriorate into a preexcited atrial fibrillation.
Acute Termination of Preexcited Atrial FibrillationPreexcited atrial fibrillation should be considered an emergency situation. If poor clinical tolerance is also present, electrical cardioversion should be performed immediately. If tolerance is acceptable, class I drugs should be administered. Classically, procainamide has been used IV, as blocking the accessory pathway will reduce the ventricular rate and produce a greater number of narrow QRS complexes.40 Flecainide administered IV is an alternative to procainamide.40 However, if treatment with this drug fails the safest option is electrical cardioversion.
Some drugs used to control the frequency of episodes of atrial fibrillation may be harmful in patients with preexcitation. Digoxin, verapamil, diltiazem, and adenosine are the most well-known potentially harmful drugs40; the observation of cases in which ventricular fibrillation occurred after the IV administration of amiodarone in patients with preexcited atrial fibrillation compels us to suggest that this drug should be ruled out in this disorder.
Long-term Treatment of Symptomatic Ventricular PreexcitationCatheter ablation is a widely used procedure that can permanently eliminate accessory pathway conduction with an efficacy greater than 90% and even more than 95% depending on the site.41 Although there may be severe complications, such as pericardial tamponade or systemic embolism, the incidence is low.41 If the site of the accessory pathway is peri-Hisian or medioseptal, there is a risk of iatrogenic AV block; this can be minimized by using cryoablation rather than radiofrequency.42
Based on these good outcomes and the association between symptomatic tachyarrhythmias (both SVT and atrial fibrillation) and sudden death in patients with ventricular preexcitation, in line with clinical practice guidelines we recommend catheter ablation as a treatment of choice in this situation.11
Drug treatment should be reserved for patients in whom ablation fails, those not considered candidates for ablation due to vascular access problems or their poor clinical condition, or patients who refuse an invasive procedure.
Long-term Treatment of Asymptomatic Ventricular PreexcitationThe therapeutic approach to patients with asymptomatic ventricular preexcitation has provoked some debate.43 A scientific approach to this problem would be to compare the risk of sudden death to the risks of the ablation procedure, considered as simple mortality or mortality plus major complications. There are no comparative studies in this regard. As mentioned, sudden death is rare in preexcitation syndromes, and if it occurs, it is typically preceded by other symptomatic tachyarrhythmias, even though exceptional cases have been described in which this is the first symptomatic manifestation.
Given the foregoing, we have reservations regarding the approach to asymptomatic preexcitation because the relationship between the risk of sudden death and the risks of the ablation procedure does not clearly favor the latter.44 However, this approach should be tempered by providing the patient with the available information; following this, if the patient prefers ablation therapy (either for personal or medical reasons), it seems appropriate to proceed with the invasive treatment. This approach is in line with the clinical practice guidelines that assign a class IIA recommendation. In addition, the patient should be made aware that immediate consultation is required if tachyarrhythmia symptoms begin because he or she would probably be a candidate for ablation. The medical history and ECG of these patients form the basis of regular clinical examinations, which should be conducted; Holter monitoring or serial echocardiograms are of little use. In contrast, if the arrhythmia presented is preexcited atrial fibrillation, this should be considered an “emergency” that requires ablation before hospital discharge.40
Long-term Treatment of Paroxysmal Tachycardias Via Concealed Accessory PathwaysThe efficacy and risks of catheter ablation are similar to those described for accessory pathways in preexcitation syndrome. As there is no risk of sudden death in these cases, the general approach and indications for ablation are similar to those described for nodal reentrant tachycardia.
Conflicts of interestNone declared.
Received 25 November 2011
Accepted 27 November 2011
Corresponding author: Unidad de Electrofisiología, Hospital Madrid Norte Sanchinarro, Oña 10, 28050 Madrid, Spain. almendral@secardiologia.es