To the Editor,
We read with great interest the scientific letter recently published in your journal by Consuegra-Sánchez et al. entitled “Increased mortality in patients with diabetes associated with olmesartan for the prevention/delay of microalbuminuria onset: a matter of concern?”1 The authors presented a meta-analysis of randomized clinical trials with placebo controls analyzing the combined effects of angiotensin-II receptor blockers (ARBs) on mortality in patients with type 2 diabetes mellitus. Without a doubt, this is an interesting study that showed the absence of all-cause risk of mortality in the diabetic population being treated with ARBs, but we would like to make a few clarifications regarding the results presented..
First, the title used poses a direct question regarding olmesartan, which invites reflection on the part of the reader, above all following the publication of the results from the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study.2 However, the combination of the results from the 5 trials analyzed, which covered 4 different drugs (irbesartan, candesartan, losartan, and olmesartan) is insufficient to respond to such a question..
The metaanalysis does not consider the effect of olmesartan (or any other ARBs) in cardiovascular mortality since it only refers to information regarding overall mortality rates, as explained by the authors. However, the authors briefly discuss the results observed in the ROADMAP study,2 which reported an increase in cardiovascular mortality (one of the primary variables evaluated) in diabetic patients treated with olmesartan..
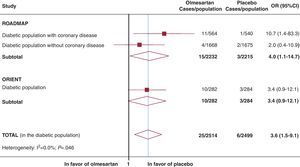
Due to the major clinical relevance of cardiovascular mortality in this population, we performed a complementary search of the clinicaltrials.gov database using the keyword “olmesartan.” The 85 results (accessed on 5 March 2012) included 2 additional completed randomized clinical trials (studies NCT00362960 and NCT00141453: Olmesartan Reducing Incidence of Endstage renal disease in diabetic Nephropathy Trial [ORIENT]).3, 4 They evaluated the effects of olmesartan on diabetic patients with or without proteinuria, renal disease, and/or cardiovascular disease. Only one of these was already published (ORIENT).3 The pooled data from the two studies (ROADMAP and ORIENT) allowed us to observe an increased cardiovascular mortality associated with olmesartan (25 cases; 1%) compared to the placebo (6 cases; 0.2%) (odds ratio: 3.6; 95% confidence interval: 1.5-9.1; Figure). It is particularly important that the risk of cardiovascular death in the ROADMAP study was 10 times higher in diabetic patients treated with olmesartan who had a history of coronary disease (Figure). In light of these data, and as a response to the question posed by Consuegra-Sánchez et al., the results from the two studies are not very promising. We must point out that approximately 49% (n=2163) of diabetic patients in the ROADMAP study had normal blood pressure and/or controlled hypertension (with at least one other cardiovascular risk factor) at the start of the study. Finally, we would like to mention that in Spain, olmesartan is authorized exclusively for the treatment of arterial hypertension.5.
Figure. Cardiovascular mortality associated with olmesartan: ROADMAP and ORIENT. In order to combine the study results, we used a fixed effects inverse variance model. We did not observe significant differences between the two studies (I2=0.0%). 95%CI, 95% confidence interval; OR, odds ratio.
NoteThe opinions expressed in this letter are the responsibility of the authors, and do not necessarily reflect the point of view of the organizations they work for..
Acknowledgements
The authors would like to thank Pilar Rayón for her collaboration and comments..
.
Corresponding author: ferran_catala@hotmail.com