Percutaneous closure of interatrial defects is an alternative to surgery that has gained acceptance in recent years to the extent that it has become the method of choice for many patients with this type of malformation. In Spain, 500 patients (children and adults) with interatrial communication (IAC) and 192 patients with patent foramen ovale (PFO) underwent percutaneous closure interventions in 2006.1
Atrial septal defects differ and sometimes prove complex.2,3 Catheterization can be used to treat ostium secundum-type, patent foramen ovale, or hybrid defects.
The key to success with the procedure lies in clear visualization of the defect, of septal remnants, and of their relation to adjacent structures. In this respect, angiography has its limitations, meaning operators must resort to echographic images of the atrial septum. These can be transthoracic, transesophageal or, more recently, intracavity—as reported in the present issue of Revista Española de Cardiología.4
Transesophageal Echography
Modern transesophageal echocardiography systems emit ultrasound waves in a 90o field perpendicular to the probe's major axis. Probes have "deflectable" tips and, moreover, ultrasound waves are electronically self-orienting from 0o to 180o. Transesophageal echocardiography provides an excellent view of the septum and adjacent structures from multiple angles but essentially 3 projections are used in daily clinical practice5: 4-chamber (superior and atrioventricular remnants), transversal aorta (retroaortic and posterior remnants), and at the level of the cavas (superior and inferior cava remnants). Currently, transesophageal echocardiography is the technique with which we have most experience and it remains the gold standard against which we should measure all new approaches to monitoring.
Intracardiac Echography
Intracardiac echocardiography provides images of the same quality as transesophageal echocardiography but from other angles. Two systems are currently available: Ultra ICE and AcuNav. The Ultra ICE system, emits in a single plane perpendicular to the catheter major axis and provides a 2-dimensional image of a 360o field in real time.4 The AcuNav system emits longitudinally to the major catheter axis in a 90o field, is currently available in 8 Fr and, moreover, includes color Doppler and a "deflectable" tip.
Transesophageal Echography Versus Intracardiac Echography
Both techniques offer a good 2-dimensional view of cardiac structures and have advantages and disadvantages.6,7 Transesophageal echocardiography requires general anesthesia and the presence of an echographer during the procedure. Intracardiac echocardiography does not require anesthesia or extra personnel. However, anesthesia is often unavoidable in treating children with IAC, regardless of the type of echographic monitoring used. We do not consider the presence of an expert echographer in the cardiac catheterization laboratory an inconvenience but, rather, valuable support. Nor does the fact the operator has to manipulate the intracavity echographic catheter seem an advantage: we prefer to concentrate on the implantation maneuvers while someone else supervises the procedure. The cost of intracavity echography catheters and the need to use femoral access also favor transesophageal echocardiography.
Septal Defect Complexity and Echographic Monitoring Type
Small IAC with good septal remnants and PFO are defects that are easy to close with simple monitoring. However, large IAC,8 doubledefects,9 or cribriform IAC3 require detailed visualization of the defects, remnants, the entire septum, and of their relations with adjacent structures. This requires a detailed echographic study and even then, incidence of procedure failure and device embolization is not inconsiderable.
In the present issue of Revista Española de Cardiología, Hernández et al analyze 52 patients with atrial septum defects receiving Amplatzer devices under intracardiac echocardiography guidance.4 They obtain very good results in treating this condition and report only 1 failure, without complications, in 1 patient. The series includes 2 patients with cribriform IAC and 4 with inadequate retroaortic remnants. In these 6 patients, intracavity echography also provided sufficient information for successful defect closure.
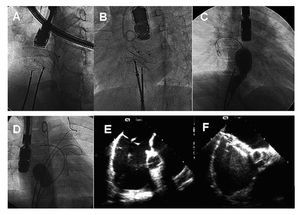
Controversy over the use of transesophageal or intracardiac echocardiography in these procedures continues since the superiority of one approach over the other has not been demonstrated. Neither technique is perfect so operators occasionally have to resort to measuring the defect with a stretching balloon (Figure). In these patients, we progressively inflate a balloon until the shunt, shown in color Doppler, disappears; we then assume this to be the maximum IAC diameter. Apart from spherical balloons (Figure, D), we also have long balloons that permit us to measure angiographically the marks that defect rims produce in the center of the balloon (Figue 1C). Discrepancies between these monitoring techniques are fully explained by Hernández et al in their discussion.4 They also comment on disadvantages of balloon measurement (excessive distension of the septum and, therefore, over-estimation of size), to which we would add the possible rupture of fine rims that sometimes are the only support to anchor the device. We consider balloon measurement useful when dealing with large, oval- or kidney-shaped defects, when the operator is not wholly sure of the maximum diameter of the orifice. In very fine septa, care is needed to avoid damaging the rims; often this maneuver can be omitted because if the size is slightly larger, part of the floppy septum gives way and adapts to the device waist.
Figure. Double IAC closure procedures in which double femoral access proves difficult. A and B: introduction of 2 devices and simultaneous verification of stability prior to release. C: after positioning a device, a second defect is measured using a balloon. D: simultaneous evaluation with 2 balloons located in different defects. E and F: transesophageal view of double IAC while the defects are being closed simultaneously with 2 devices.
A further noteworthy aspect of complex defect procedures with intracavity echography monitoring is the need for access via both femoral veins: one for the device and the other for the echography. In contrast, transesophageal monitoring leaves one femoral vein free for other uses (Figure), such as introducing 2 stretching balloons, a second stretching balloon and a device, or 2 devices in patients with multiple IAC, meaning the procedure can be completed in a single intervention (Hernández et al reported 1 patient required a second procedure4). This strategy facilitates simultaneous testing of device stability (Figure) before definitive release. Contralateral femoral access also permits the introduction of a second catheter to correct perpendicular presentation of the occluder10 in patients with a small left atrium and large IAC.
Looking to the Future
No perfect monitoring technique, providing precise visualization of the full extent of the defect, currently exists. Mentally, we have to integrate 2-dimensional information to reconstruct a 3-dimensional structure (the atrial septum). We can only hope that a means of mapping defects exactly or of simultaneously visualizing all possible orifices, their diameters and the distances between them will come available in the future. This would enable us to plan occluder(s) size and avoid the errors we currently commit when estimating it. These errors lead to implantation failure (with the corresponding costs) or complications due to device embolization.11 In our series, this occurred in 7 (2.6%) of 272 patients monitored by transesophageal echocardiography, a figure similar to that published elsewhere.12 New generations of echographs with 3-dimensional reconstruction systems, or new views through future radiologic systems (multislice computerized tomography or magnetic resonance) that improve on the features of current models, will simplify and provide greater safety in these percutaneous techniques. In any case, articles like that by Hernández et al4 forge a path towards new views that serve as an alternative to classic transesophageal echocardiography.
Correspondence:
Dr. M. Pan.
Servicio de Cardiología. Hospital Universitario Reina Sofía.
Avda. Menéndez Pidal, 1. 14008 Córdoba. España.
E-mail: manuelpan@telefonica.net