Bariatric surgery is a valuable tool for metabolic control in obese diabetic patients. The aim of this study was to determine changes in weight and carbohydrate and lipid metabolism in obese diabetic patients during the first 4 years after bariatric surgery.
MethodsA retrospective study was performed in 104 patients (71 women; mean age, 53.0 [0.9] years; mean body mass index, 46.8 [0.7]) with type 2 diabetes mellitus (median duration, 3 years) who underwent laparoscopic proximal gastric bypass.
ResultsBlood glucose levels and glycated hemoglobin concentrations decreased during the first 1-3 postoperative months. Values stabilized for the rest of the study period, allowing hypoglycemic treatment to be discontinued in 80% of the patients. No significant differences were observed as a function of the body mass index, diabetes mellitus duration, or previous antidiabetic treatment. Weight decreased during the first 15-24 months and slightly increased afterward. Levels of total cholesterol, triglycerides, and low-density lipoprotein significantly decreased, and target values were reached after 12 months in 80% of the patients. No correlation was found between these reductions and weight loss. Similarly, high-density lipoprotein concentrations decreased until 12 months after surgery. Although concentrations showed a subsequent slight increase, target or lower high-density lipoprotein values were achieved at 24 months postintervention in 85% of the patients.
ConclusionsBariatric surgery is effective for the treatment of obese diabetic patients, contributing to their metabolic control and reducing their cardiovascular risk.
Keywords
Obesity is a chronic and multifactorial disease characterized by an excess of body fat that results in pathological weight gain. This disease affects a considerable proportion of the population, encompassing both sexes and all ages and social classes. It is estimated that the prevalence of persons with a body mass index (BMI)>30 doubled between 1986 and 2000. In addition, the proportion of people with a BMI>35 has reached 15.5% of the population of the United States.1,2 Similarly, it has been calculated that the prevalence of morbid obesity has increased by more than 200% in Spain.3
A parallel increase has been seen in the incidence of type 2 diabetes mellitus (T2DM) in recent decades. The pathogenesis of this disease is based on insulin resistance, which is tightly linked to overweight and obesity; these conditions are frequent in T2DM patients and–in particular–abdominal obesity is considered the main risk factor for T2DM. In a recent study published in Spain, at least 30% of the population showed some type of alteration in carbohydrate metabolism, with an age- and sex-adjusted prevalence of DM of 13.8%.4
Current noninvasive treatments for obesity, which include medication, produce modest results that show poor long-term maintenance.5,6 The unsatisfactory results obtained by noninvasive approaches are especially notable for T2DM patients, who struggle to achieve overall metabolic control, which is characterized by a decrease in glycated hemoglobin A1c (HbA1c) and blood pressure and a long-term improvement in lipid profile, as shown by some studies recently published in Spain.7,8
Another treatment strategy for these syndromes is bariatric surgery. This approach attempts to modify the anatomy of the digestive system in order to reduce gastric capacity, either alone or in combination with variable degrees of intestinal malabsorption, so as to reduce body weight and improve associated comorbidities.9–12 These surgical techniques have been gradually refined. A major advance was made in 1994 when Wittgrove and Clark performed the first laparoscopic gastric bypass, significantly improving the safety of the procedure and reducing the surgical trauma associated with the operation.5 Since then, the number of interventions performed has increased exponentially; it is estimated that 344 000 operations of this type were performed worldwide in 2008, 5000 in Spain alone.13
Bariatric surgery has proven to be extremely useful for obese patients with T2DM, not only for reducing body weight, but also for improving glycemic control. The mechanism involved remains to be clarified, although several theories have attempted to provide an explanation.14 Patients also show improvements in blood pressure, lipid profile, and sleep apnea, among other complications often associated with obesity.15
This article describes the results obtained in a 4-year retrospective study carried out in a general hospital into the efficacy of bariatric surgery in improving glycemic control in patients with obesity and T2DM.
METHODSThis was a retrospective study of 104 patients with a BMI>35 (mean, 46.8 [0.7]) who also had T2DM (71 women and 33 men; mean age, 53.0 [0.9] years; duration: median, 3 years; mean, 9.2 [0.9] years; range, 1-23 years) according to the diagnostic criteria of the American Diabetes Association.16 All patients met the surgical criteria of the National Institutes of Health.17
The patients underwent bariatric surgery between 2002 and November 2011 (gastric band surgery in 11 and laparoscopic proximal gastric bypass in 93). All patients had previously been assessed in the obesity unit of the department of endocrinology and nutrition of our hospital, which includes endocrinologists, nutritionists, surgeons, psychiatrists, and rheumatologists. Patient confidentiality was guaranteed through the use of code numbers. All patients were fully informed about the methods being used and provided written consent in accordance with national laws and the Declaration of Helsinki, 2000 revision (Declaration of Edinburgh).
Patients with type 1 DM or other etiologies were excluded, as were those with characteristics that contraindicated bariatric surgery: age<18 or >60 years, secondary diseases that explained the obesity, drug or alcohol abuse, major psychiatric disorders (eg, schizophrenia, psychosis), intellectual disability, eating disorders (eg, bulimia nervosa), and pregnancy or the desire to become pregnant in the year following surgery.
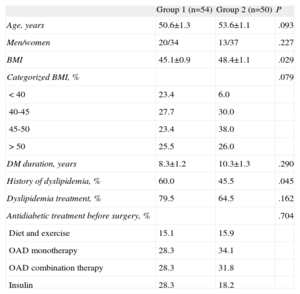
All patients received intensive weight control treatment and followed a diet plan and a personalized physical exercise regimen, both before and after surgery. Patient data were collected before the operation and 1, 3, 6, 12, 15, 24, 36, and 48 months afterward. Mortality was nil. Follow-up was irregular: some patients missed some of the clinical visits and not all patient parameters were measured during the visits. Thus, the mean follow-up was 36.57 months and 54 patients had 4-year follow-up data (only 1 of these underwent gastric band surgery). To analyze the results based on follow-up, 2 groups were created: group 1, consisting of the 54 patients who completed the 4-year follow-up; and group 2, made up of 50 patients who did not complete this follow-up. No differences between the 2 groups were observed with respect to age, sex distribution, or DM duration or treatment. Group 1, which completed the 4-year follow-up, showed a lower presurgical BMI than group 2, but no differences were seen when the groups were stratified by BMI. In addition, group 1 showed lower dyslipidemia, but the proportion of patients receiving hypolipidemic therapy was similar (Table 1). The patients also received the following hypoglycemic, antihypertensive, and hypolipidemic therapy according to the recommendations of the American Diabetes Association to achieve the following target values: HbA1c<7%, low-density lipoprotein (LDL)<100mg/dL, high-density lipoprotein (HDL)>40mg/dL for men and >50mg/dL for women, triglycerides<150 mg/dL, systolic blood pressure≤130 mmHg, and diastolic blood pressure≤80 mmHg.
Descriptive Characteristics of the Sample by Completion of a 4-Year Follow-up (Group 1) or not (Group 2)
| Group 1 (n=54) | Group 2 (n=50) | P | |
| Age, years | 50.6±1.3 | 53.6±1.1 | .093 |
| Men/women | 20/34 | 13/37 | .227 |
| BMI | 45.1±0.9 | 48.4±1.1 | .029 |
| Categorized BMI, % | .079 | ||
| <40 | 23.4 | 6.0 | |
| 40-45 | 27.7 | 30.0 | |
| 45-50 | 23.4 | 38.0 | |
| >50 | 25.5 | 26.0 | |
| DM duration, years | 8.3±1.2 | 10.3±1.3 | .290 |
| History of dyslipidemia, % | 60.0 | 45.5 | .045 |
| Dyslipidemia treatment, % | 79.5 | 64.5 | .162 |
| Antidiabetic treatment before surgery, % | .704 | ||
| Diet and exercise | 15.1 | 15.9 | |
| OAD monotherapy | 28.3 | 34.1 | |
| OAD combination therapy | 28.3 | 31.8 | |
| Insulin | 28.3 | 18.2 |
BMI, body mass index; DM, diabetes mellitus; OAD, oral antidiabetic.
Unless otherwise indicated, data are expressed as mean±standard deviation.
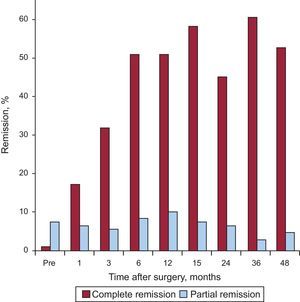
Complete remission of DM was defined as a decrease in plasma fasting glucose concentrations to <100 mg/dL and of HbA1c to <6%. In addition, remission was considered partial when fasting glucose was between 100 and 125 mg/dL and HbA1c was <6.5%.
The primary outcome variables evaluated were fasting glucose, HbA1c, weight, and BMI (weight in kilograms/height in meters squared). The secondary outcome variables evaluated were total cholesterol, LDL cholesterol (LDL-C) (calculated with the Friedewald formula),18 HDL cholesterol (HDL-C) and total triglycerides.
The sample was divided into various groups according to BMI (4 groups: 35-40, 40-45, 45-50, and >50), the duration of T2DM (2 groups: <10 years and >10 years), and presurgical antidiabetic therapy (4 groups: diet and exercise, antidiabetic monotherapy, antidiabetic combination therapy, and insulin therapy).
The following methods were used to obtain the different measurements: UV-hexokinase for fasting glucose, homogeneous immunoassay for HbA1c, precipitation with phosphotungstic acid, CHOD-PAP and magnesium ions/HDL-C and total cholesterol, and the GPO-PAP colorimetric enzymatic method for triglycerides. All biochemical analyses were performed after a 12-h fast with a Hitachi 747-734 analyzer (Boehringer Mannheim).
Data analysis was performed with the freely available software package R2.1519 (www.r-project.org).19 Longitudinal analysis was used to compare the means and proportions (where applicable) of each visit with the previous one. Comparison of the changing patterns of HbA1c in the distinct groups (BMI, DM duration, and therapy type) was performed through the L2 distance between the resulting curves. All P values were calculated with the general bootstrap method,20 which allows all the data to be used even when data are missing.21 A P value of <.05 was considered statistically significant. Spearman correlations were also performed. Numerical variables are expressed as means (standard error). Categorical variables are expressed as relative frequencies.
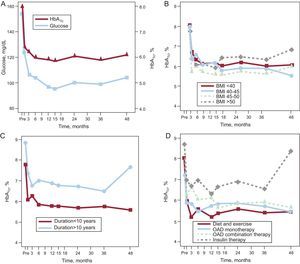
RESULTSGlycemic ControlAs shown in Figure 1A, mean fasting glucose values decreased from more than 150 to about 120 mg/dL in the first postoperative month (P<.001), reaching 105 mg/mL in the third month (P=.011). Normal glucose values were subsequently maintained during the remaining 4 years of the study. The concentrations of HbA1c also decreased after the first month (P<.001) and were largely stable for the remainder of the evaluation time. These changes correlated well with the rapid weight loss that occurred during the first month (r=0.473; P=.007) for the fasting glucose level, but not for HbA1c. No such correlation was seen in subsequent months because, although the weight loss continued, the plasma values had normalized and were stable. An analysis of the correlation between the decrease in HbA1c concentrations and the patients’ previous pathological characteristics revealed no relationship with BMI (Fig. 1B). With regard to DM duration (about 10 years), patients, with a longer duration showed a greater decrease in the first month (P=.029), although both groups subsequently showed a similar pattern (Fig. 1C). Similarly, although we found differences in the decrease in HbA1c based on the presurgical antidiabetic therapy (P=.012), subsequent changes were similar in all 4 groups (Fig. 1D). However, 4 years after surgery, patients with a T2DM duration >10 years who had received insulin therapy preoperatively showed worse metabolic control, although this difference was not statistically significant.
Changes in blood glucose and glycated hemoglobin A during the 4 years after surgery (A), correlation between changes in glycated hemoglobin A and body mass index (B), type 2 diabetes mellitus duration (C), and hypoglycemic therapy before surgery (D). BMI, body mass index; HbA1c, glycated hemoglobin A1c; OAD, oral antidiabetic.
The complete remission rate of T2DM increased significantly from the first postoperative month, reaching a maximum value of about 60% of the patients at 3 years. The number of patients with partial remission gradually increased until the 12th postsurgical month, when it reached 10.3%; subsequently, the values decreased due, at least in part, to the incorporation of many of these patients to the complete remission group (Fig. 2).
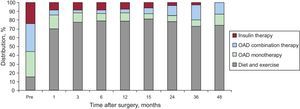
The need for hypoglycemic therapy markedly decreased after surgery (Fig. 3). The numbers of patients not requiring medication progressively increased until, at 15 postoperative months, more than 80% did not need any type of drug treatment in addition to their diet and physical exercise regimen. Subsequently, requirements for oral antidiabetics increased, either alone or in combination. The number of patients that could dispense with the use of exogenous insulin progressively increased, and 4 years after surgery, none of our patients required insulin.
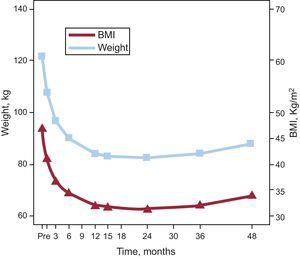
Weight ControlBody weight significantly decreased from months 1 to 12 after surgery (P<.001), reaching a minimum after 15-25 months, and remaining stable thereafter, with a mean decrease of 40kg. BMI decreased in parallel with weight, reaching a median value consistent with grade I obesity at 24 months. At this time, 20.6% (n=22) and 7.5% (n=8) of the patients had BMIs <30 and <25, respectively. Thereafter, a moderate weight recovery of 8kg was seen, which translates to a BMI increase of 2.5 points (Fig. 4).
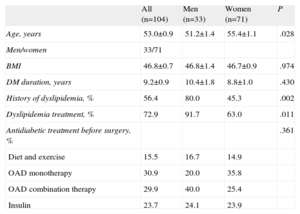
Lipid ControlIn our cohort, 56.4% of the patients had some degree of dyslipidemia as a complication associated with their clinical profiles. Of these, 72.6%, the majority male, were receiving some type of lipid lowering therapy (Table 2).
Descriptive Characteristics of the Total and Sex-Stratified Sample
| All (n=104) | Men (n=33) | Women (n=71) | P | |
| Age, years | 53.0±0.9 | 51.2±1.4 | 55.4±1.1 | .028 |
| Men/women | 33/71 | |||
| BMI | 46.8±0.7 | 46.8±1.4 | 46.7±0.9 | .974 |
| DM duration, years | 9.2±0.9 | 10.4±1.8 | 8.8±1.0 | .430 |
| History of dyslipidemia, % | 56.4 | 80.0 | 45.3 | .002 |
| Dyslipidemia treatment, % | 72.9 | 91.7 | 63.0 | .011 |
| Antidiabetic treatment before surgery, % | .361 | |||
| Diet and exercise | 15.5 | 16.7 | 14.9 | |
| OAD monotherapy | 30.9 | 20.0 | 35.8 | |
| OAD combination therapy | 29.9 | 40.0 | 25.4 | |
| Insulin | 23.7 | 24.1 | 23.9 |
BMI, body mass index; DM, diabetes mellitus; OAD, oral antidiabetic.
Unless otherwise indicated, data are expressed as mean±standard deviation.
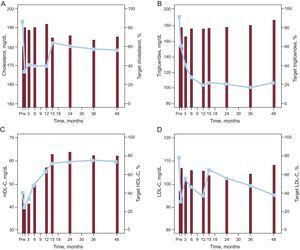
Figure 5A shows changes in total blood cholesterol concentrations after gastric bypass. Total blood cholesterol concentrations rapidly decreased from the first month (P<.001), and this decrease was maintained throughout the remainder of the first year. These concentrations became normalized in more than 80% of the patients and reached target values for patients with DM. At 15 months, total cholesterol concentrations increased slightly (P=.009) and stayed at these levels during the 4 years after surgery. Despite this delayed increase, the target total cholesterol level was reached in approximately 70% of the patients 4 years after gastric bypass.
Changes in concentrations of total cholesterol (A), triglycerides (B), high-density lipoprotein cholesterol (C), and low-density lipoprotein cholesterol (D) during the 4 years after surgery (lines) and the percentage of patients (histograms) that met or exceeded the target values for diabetics with respect to each of the parameters analyzed. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Blood triglyceride concentrations markedly decreased during the first year (P=.008). The triglyceride level subsequently stabilized at about 100mg/dL (fourth year mean, 106.5 [8.7] mg/dL), which is much lower than 150mg/dL, the maximum target value for patients with DM. Correspondingly, a rapid but insignificant increase was seen in the proportion of patients who reached the target level, increasing from about 80% 6 months after the surgery to 88.9% after 4 years (Fig. 5B).
Plasma HDL-C concentrations decreased during the first postoperative month (P<.001) and then progressively increased until month 15 (P=.004). Thereafter, HDL levels stabilized at about 60mg/dL. This reduction in HDL-C levels was reflected in the proportion of patients reaching the HDL target value (>40mg/dL for men and >50mg/dL for women), which decreased from 42.2% before surgery to less than 30% at 3 months. HDL concentrations subsequently gradually increased until the 15th month (P=.026) before stabilizing at around 80% to 85% (Fig. 5C).
The mean LDL-C concentrations were more variable but were still lower than before surgery. Levels significantly decreased in the first postoperative month and this decrease was maintained until month 15. At that time, the values increased (P=.027), staying at this level until the end of follow-up. Consequently, the number of patients that managed to maintain their LDL values below the target for diabetics (<100mg/dL) after surgery increased from 30% to between 55% and 60%, with a maximum of 68% at 4 years; these changes in percentages did not reach statistical significance (Fig. 5D).
The variations in the total cholesterol, triglycerides, and LDL-C did not correlate with postoperative weight loss. However, a relationship was observed with HDL-C concentrations after 2 years (24 months, r=–0.436; P=.007; 36 months, r=–0.522; P=.004).
Accordingly, the number of patients receiving lipid lowering therapy decreased to 17.8%, 15.6%, 14.9%, 10.0%, 15.3%, 20.0%, 20.5%, and 19.0% (all P<.05) after 1, 3, 6, 12, 15, 24, 36, and 48 months, respectively.
DISCUSSIONIn addition to the expected benefits, bariatric surgery afforded patients unforeseen and seemingly unrelated resolution of their T2DM. Although the first data were obtained in the 1970s, confirmation of this effect of bariatric surgery has been more recent. Special mention must go to Pories et al., whose 10-year retrospective analysis presented data showing DM remission in patients who underwent bariatric surgery. It was also clear from this study that the effect on blood glucose appeared in the first few days after surgery, that is, before the start of weight loss.22
Despite these results, few randomized controlled studies have aimed to evaluate bariatric surgery as a treatment for T2DM. In the current study, we found a marked reduction in glucose and HbA1c levels in the first month after surgery, allowing hypoglycemic medication to be significantly reduced. The improvement in glucose metabolism did not correlate with weight loss, except in the first month, although other variables related to the surgical procedure (eg, fasting, food stage, postsurgical blood loss) may have played a role during this period. In contrast, hematologic values continued to improve for the remainder of the first postsurgical year and were maintained during the rest of the period studied. We observed no relationship between the rate of the decrease in HbA1c and the presurgical BMI, and only those groups with a longer T2DM duration or who underwent presurgical insulin therapy showed some deterioration. The significance of this disimprovement is unclear, however, as it was only evident at the last follow-up evaluation. Nonetheless, even in these patients, management of the diabetes was easier than before the surgery, as shown by the fact that none of the patients required insulin therapy 4 years after surgery.
These results are consistent with those of other observational studies, such as that of Dixon et al.23 (2008), which found that patients with an adjustable gastric band showed greater glycemic control than those who followed conventional treatments (lifestyle changes and hypoglycemic therapy). Similarly, Mingrone et al.,24 who studied the progress of patients who underwent proximal gastric bypass, biliopancreatic diversion, or conventional therapy, found that all 3 groups showed improvements in HbA1c levels at the 1-year follow-up. The 2 surgical groups, however, showed greater HbA1c reductions, particularly the biliopancreatic diversion group. Moreover, the remission rate of T2DM was 0% in the conventional therapy group, 75% in the gastric bypass group, and 95% in the biliopancreatic diversion group. Like us, these authors also observed that age, sex, presurgical BMI, DM duration, and changes in body weight were not predictive factors for T2DM remission. Finally, Schauer et al.25 found that the rate of T2DM remission in patients who underwent conventional therapy was 12% compared with 42% and 37% in those who underwent gastric bypass and sleeve gastrectomy, respectively. These authors found no relationship between improved glycemic control and weight loss.
The glycemic control data were accompanied by such an improvement in the lipid profile that a high percentage of the patients reached or bettered the target figures for triglycerides, LDL-C, and HDL-C. Our data are consistent with those of previous studies that found normalization of dyslipidemia in up to 96.9% of gastric bypass patients,14 which was accompanied by a significant reduction in the risk of having a cardiovascular event due to the decrease in associated atherogenic dyslipidemia.
The changes observed after surgery are a consequence of a cascade of events that starts with up to a 4- or 5-fold increase in insulin sensitivity, partly due to an increase in the concentrations of adiponectin that accompanies the decrease in adipose tissue, particularly in the liver and muscle. In muscle, the number of insulin receptors and lipid metabolism increase, reducing the concentration of myocyte lipids and increasing glucose uptake. In parallel, the fatty acid content of the liver is reduced, which is accompanied by a decrease in fatty liver-associated insulin resistance. All these events are accompanied by an increase in insulin secretion, and it has been postulated that the recovery of the incretin response could be crucial.26–31
LimitationsThe current study has a number of limitations resulting from its retrospective study design, such as irregular follow-up and missing data. Moreover, data on hospital stay, reoperations, and postsurgical morbidity, including the phenomenon of dumping, were not collected, as they were not objectives of the current study, which could have affected the final result. Moreover, most patients underwent laparoscopic proximal gastric bypass and only 11 were treated with an adjustable gastric band. Accordingly, to determine the full impact of surgery, it would be necessary to include patients treated with other surgical techniques, such as sleeve gastrectomy and biliopancreatic diversion.
CONCLUSIONSIn this study, we have presented data showing that gastric bypass is an effective procedure for the treatment of obese patients with a history of T2DM. Not only did we observe significant weight loss, but the glycemic control achieved was greater than expected and was evident even before the weight loss. Surgery also helped to control the altered lipid levels frequently associated with morbid obesity, which improved the patients’ general cardiometabolic profile and helped them reach good control targets.
CONFLICTS OF INTERESTNone declared.