For many years, cardiac catheterization and angiocardiography have been the main diagnostic methods for morphological and functional assessment of congenital shunts. However, catheterization techniques are invasive, require injection of iodide contrast and exposure to x-rays, and complications may arise, particularly in pediatric patients. In the last 2 decades, echocardiography has progressively displaced invasive methods in the overall evaluation and definitive diagnosis of children and adults with congenital heart diseases.1 Although echocardiography is a safe, cheap technique that offers high diagnostic yield, it also has important limitations. The echocardiographic image is less clear in older children and adults than in infants and small children, in whom high-frequency transducers with high spacial resolution are required. In addition, multiple surgical interventions in older children and adults may cause further deterioration of the image. Although echocardiography provides high anatomic definition of almost any cardiac structure, it is virtually blind to what is happening outside the heart, a few centimeters into the vascular system. This technique is particularly limited in assessment of pulmonary or systemic venous drainage or peripheral pulmonary artery circulation. It is also very imprecise in evaluation of the thoracic aorta, congenital or surgical fistulas, or collateral systemic vessels. Echocardiography is even more limited in functional assessments. The size and function of the left chambers can be readily assessed by 2-dimensional echocardiography, but this technique is much less useful in the evaluation of the right chambers. Right ventricular function is particularly difficult to determine by echocardiography, but in congenital heart disease, the right ventricle may be of similar if not more importance than the left one, particularly when it is the systemic ventricle. Cardiac echo-Doppler imaging provides an excellent quantitative assessment of valvular stenosis and a good qualitative or semiquantitative assessment of valve regurgitation and cardiac shunts, but once again the value of the technique is much more limited when obstructions and shunts are located outside the heart. In addition, many surgical indications depend on accurate quantification of shunts, but quantification of congenital shunts remains one of the most important limitations of Doppler echocardiography. Although pulmonary systolic pressure can be determined accurately by applying the Bernoulli equation to the peak velocity of the regurgitation jet, this approach cannot accurately determine the mean pulmonary artery pressure, pulmonary vascular resistance, or response to oxygen and the vasodilators used in the therapeutic indications of congenital heart disease in patients with severe pulmonary hypertension due to systemic-pulmonary shunts.
Morphological Assessment by Cardiac Magnetic Resonance Imaging
In recent years, magnetic resonance imaging (MRI) has emerged as a diagnostic technique of growing importance in the assessment of congenital heart diseases in children and adults.2 Morphological and functional assessment of congenital shunts can be readily made with this technique and it is particularly sensitive in certain aspects for which echocardiography provides insufficient information. MRI is not limited as echocardiography by the acoustic window, and so the image is not affected by chest size or previous operations. Nevertheless, it is also subject to its own limitations, for example, it cannot be used in patients with pacemakers or defibrillators, arrhythmias, or claustrophobia, and it may be subject to respiratory artifacts (if the subjects are unable to hold their breath) or the need for sedation or intubation in small children or patients unable to cooperate.
Electrocardiogram-gated spin-echo and gradient-echo cine sequences provide basic morphologic information.3 These techniques allow an accurate definition of the intra- and extracardiac anatomy and compete favorably with echocardiography in many aspects. They are particularly useful for determining visceral-atrial situs, connection and course of great veins and arteries, certain septal defects that may be hidden in echocardiography (such as atrial, sinus venosus, and supracristal, or posterior ventricular septal defects), anatomic definition of the right ventricular outflow tract, the origin and proximal course of the coronary arteries, and, above all, assessment of the size and function of the cardiac chambers, and in particular the right ventricle.4 In many patients who undergo operations using atrial baffles to redirect flow, atriopulmonary, or cavopulmonary anastomosis, and placement of prosthetic conduits or large patches for extension of the outflow tracts or the aortic isthmus, MRI has become an irreplaceable technique for long-term monitoring and early detection of complications.
Another possibility offered by MRI is angiographic MRI with intravenous contrast (gadolinium). This method provides a 3-dimensional image with a wide field of vision that shows the course and morphology of the vessels that lie outside the area seen by echocardiography.5,6 The technique is particularly useful for demonstrating pulmonary venous drainage patterns, anomalies in the pulmonary arteries, anatomy of the thoracic aorta, surgical fistulas, or systemic-pulmonary collateral vessels. The use of gadolinium as a contrast also allows detection of late enhancement of the myocardial wall due to fibrosis or necrosis resulting from infarction or the effect of prior surgical procedures.7
Magnetic Resonance Imaging With Phase Contrast
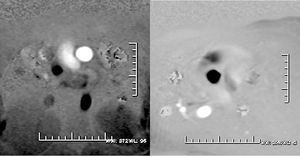
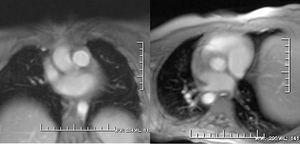
MRI has not been left behind in the functional assessment of obstructive lesions, valve regurgitations, and quantification of shunts. For these purposes, phase contrast or flow mapping is the main approach.8 The method used is based on the principle that the change in phase of protons moving in a magnetic field is proportional to the velocity of flow. Signal from stationary tissue is suppressed using a bipolar gradient and the spins of moving protons progressively relax (changing their angular phase) as a function of velocity. Longitudinal projections along the direction of vessel or jet are difficult to quantify and serve for a qualitative assessment of the direction of the jet and the length of the shadow. Thus, for volumetric calculations, the sequence is programmed in cuts perpendicular to the vessel or jet under study. The maximum expected velocity from the specific flow is defined (usually 150 cm/s in the pulmonary artery and 250 cm/s in the aorta) and 2 types of images are obtained: phase and magnitude. Phase images are a pixel combination in gray tones that vary according to the intensity of the signal (white-black) and the phase of the cardiac cycle (systole or diastole) (Figure 1). Magnitude images are similar to those obtained in the morphologic study with sequences of contrast-enhanced blood (Figure 2). The device overlays the area of interest semiautomatically on top of these phase images, and values are obtained corresponding to the vessel area, peak velocity, total flow, beat-to-beat volume, and anterograde and retrograde flow. Instantaneous measurements taken at multiple points during the cardiac cycle are plotted against time to build up a flow curve. The integral of the area under curve determines the flow volume during the cardiac cycle. The anterograde volume is determined by the part of the curve above the baseline and the retrograde volume by the curve below baseline. Knowing the peak and mean flow velocity also allows the pressure gradients to be calculated by means of the modified Bernoulli equation, as is commonplace with cardiac Doppler ultrasound. We should bear in mind that these sequences last twice as long as the normal ECG-gated cinematic sequences and that the patients must hold their breaths for longer; likewise, a unique bipolar gradient can give erroneous velocities if the magnetic field is not homogeneous.9
Figure 1. Phase images with oblique projections to obtain a view perpendicular to the direction of the pulmonary artery (left) and the aorta (right). A "circle" of white signal (pulmonary artery) or black signal (ascending aorta) can be seen, depending on the direction of flow. The region of interest is overlaid on top of these circles to measure the velocity and flow in each vessel.
Figure. 2. Magnitude images obtained in the same view as the phase images. These images allow the anatomic site of the structures under study to be determined.
This technique is used for functional assessment of aortic coarctation (by measuring collateral flow and the pressure gradient across the aortic coarctation), pulmonary branch lesions (by measuring the differential flow between the 2 branches or the pressure gradient in vascular stenoses), assessment of valve lesions (by determining the regurgitation jet or pressure gradient), or obstruction of the conduits, cardiac baffles, surgical fistulas, or venous shunts. But its most important application is in quantifying shunting.
Quantification of Shunting and Pulmonary Pressure
The basic technique consists of simultaneously measuring blood flow in the ascending aorta and the main pulmonary artery, thereby allowing determination of the pulmonary flow (PF), systemic flow (SF), and the ratio PF:SF. Many studies have shown the precision of this technique in quantifying shunts in adults and adolescents.10,11 In this issue of Revista Española de Cardiología, Hernández-González et al12 present their experience in a pediatric population with congenital shunts and pulmonary artery hypertension. The authors report an excellent correlation with cardiac catheterization for pulmonary load, systemic load, and flow ratios, but MRI tended to underestimate both the pulmonary and systemic flow compared to invasive catheterization. Underestimation of the flow may occur if the target vessel is not examined in a direction completely perpendicular to the direction of flow or if the peak velocity selected is lower than the peak velocity at any time during the cardiac cycle. Moreover, in very small children, with small vessels, the reliability of the method decreases because the number of pixels can be very low. Alternatively, the difference between MRI and the Fick method may also be due to different conditions in children during cardiac catheterization and MRI or an overestimation of the data obtained in the catheterization study. In any case, this study confirms how useful the method can be in assessing shunts when applied to a pediatric population with a high suspicion of pulmonary hypertension.
However, the intraclass correlation between MRI and catheterization in the assessment of pulmonary systolic pressure was much lower, with a substantial underestimation of the pulmonary pressure with MRI. In this case, the methodological limitations could be much greater. Unlike cardiac Doppler ultrasound, to determine the peak velocity of the tricuspid regurgitation jet, this jet has to be measured perpendicularly to motion in the area of peak velocity. This might, however, be very difficult to achieve because the atrioventricular valve plane is in constant motion during the cardiac cycle. Moreover, the tricuspid valve plane is very different to that of the mitral valve and it may be very difficult to trace this plane in patients with pulmonary hypertension and ventricular dilation in a single slice. This all contributes to a decrease in spacial resolution which, added to the limited time resolution of the technique, may lead to substantial underestimation of the peak flow velocity. Thus, determination of pulmonary systolic pressure using this method may be less reliable than with the Doppler method. In any case, both methods--cardiac Doppler and MRI with phase contrast--share the same limitations. So although morphological indexes have been described13 and phase contrast techniques have been published that can estimate mean pulmonary artery pressure or pulmonary vascular resistance with MRI,14 cardiac catheterization continues to be the gold standard for evaluating pulmonary hemodynamics.15
Conclusions
Doppler echocardiography is currently the most widely used technique for morphological and functional assessment of congenital shunts. Nevertheless, MRI is an emerging diagnostic technique that offers complete information in patients with a poor acoustic window. In certain septal defects, such as atrial shunts of the venous sinus, uneven supracristal ventricular shunts, or endocardial cushion defects, the morphological information obtained by MRI may be superior to that obtained with echocardiography. MRI also has an incremental value in the assessment of the size and function of the right ventricle or long-term follow-up of complex congenital heart diseases that have been surgically corrected. In patients with extracardiac shunts, particularly abnormalities in pulmonary venous drainage, surgical fistulas, or systemic collateral vessels, angiographic MRI has already become the diagnostic technique of choice. When quantification of shunts becomes a key aspect in the indication for surgery, assessment by phase contrast MRI is a strong alternative to invasive studies. However, in patients with severe pulmonary hypertension related to congenital systemic-pulmonary shunting, therapeutic, surgical, or pharmacological decisions still need right cardiac catheterization to accurately determine pulmonary pressure, vascular resistance, and response to oxygen and vasodilators.
See article on pages 907-13
Correspondence: Dr. J.M. Oliver Ruiz.
Unidad de Cardiopatías Congénitas del Adulto.
Hospital Universitario La Paz.
P.o de la Castellana, 261. 28046 Madrid. España.
E-mail: joliver.hulp@salud.madrid.org