To the Editor:
Inborn errors of beta-oxidation metabolism, known as fatty acid oxidation defects (FAODs), constitute a relatively new group of recessive autosomal genetic diseases, and various types and subtypes have been described. The true incidence of this condition is probably underestimated because many cases go unnoticed.
Fatty acid beta-oxidation represents an important source of energy, particularly in situations of fasting and metabolic stress (prolonged exercise, infections, fever, exposure to cold). Once glycogen deposits have been depleted, fatty acids are mobilized from the adipose tissue to provide the glucose energy effect. This pathway is particularly important in the first 2 days of life.1 The heart, skeletal muscle, and liver are highly dependent on the pathway.
The clinical spectrum and prognosis of FAODs are extremely variable, because they depend on the enzyme deficiency and patient age; there have been reports of cases with few or mild symptoms, as well as patients with severe conditions. A common characteristic of all these cases (except those involving short-chain and, on occasions, medium-chain) is hypoketotic hypoglycemia with fasting. Other symptoms are muscle pain, hypotonia, peripheral neuropathy, hepatopathy, dilated or hypertrophic cardiomyopathy, cardiac arrhythmias, and sudden death.1
We describe the case of a 15-year-old adolescent girl with diffuse muscle disease and peripheral neuropathy who presented with difficulty walking, but no cardiologic symptoms. She had been referred to the cardiology outpatient clinic for long-chain 3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD) deficiency diagnosed at age 1 due to hypoketotic hypoglycemia in metabolic stress, hepatopathy, and pigmentary retinopathy. The condition was managed by diet with restriction of long-chain fats (10%), addition of medium-chain triglycerides (20%) of total caloric intake, fat-soluble vitamin supplements, and avoidance of fast periods over 8 h.
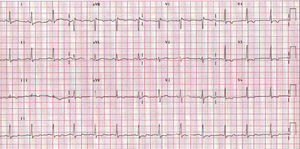
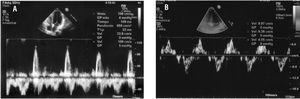
The physical examination was normal. Electrocardiography showed sinus rhythm with right bundle-branch block and negative T wave on the lateral aspect, in the probable context of systolic overload (Figure 1). The echocardiogram revealed good ventricular function with 57% ejection fraction, no evidence of ventricular hypertrophy (interventricular septum, 7 mm; posterior wall, 8 mm), and no valvular condition or pericardial effusion. The transmitral Doppler flow study showed E wave of 1.06 m/s, A wave of 0.33 m/s, and pressure half-time of 110 ms (Figure 2A). Doppler tissue imaging of the mitral annulus velocity during Valsalva's maneuver2 found S' of 8.97 cm/s, E' of 4.05 cm/s, and A' of 9.36 cm/s (Figure 2B).
Figure 1. Electrocardiogram.
Figure 2. Doppler patterns.
The diagnosis is based on the levels of acylcarnitines, acylglycines, organic acids, carnitine, free fatty acids, and 3-hydroxy acids, in conjunction with enzymatic and genetic studies. The determination of urine organic acids and plasma acylcarnitines may not differentiate LCHAD deficiency from trifunctional protein deficiency and, therefore, the diagnosis can only be confirmed by identifying the G1528C mutation (present in 90% of mutated alleles, as in our case) or measuring enzyme activity (also confirmed in this patient). On a few occasions, challenge testing is required.3
Cardiac involvement is observed in 50% of patients with FAODs. Hypertrophic cardiomyopathy tends to be the most common form of presentation (60%) followed by dilated cardiomyopathy (27%); echocardiography is the most useful diagnostic method.4
Rhythm abnormalities are also common and may present in the form of blockades (bundle-branch block, atrioventricular node block, sinus node dysfunction), ventricular arrhythmia (tachycardia, ventricular fibrillation), or supraventricular arrhythmia.4 Therefore, it is extremely important that the differential diagnosis in newborn arrhythmias include FAODs because long-chain acylcarnitine accumulation at the cardiac level can induce arrhythmias.5
The basic treatment in these patients consists of avoiding periods of fast and restricting fat intake through increased carbohydrates.6
The case presented in a patient with an FAOD (LCHAD) associated with diastolic dysfunction with restriction and high filling pressures, may indicate acylcarnitine accumulation in the cardiac tissue.