To the Editor:
Percutaneous closure of ostium secundum atrial septum defects is now accepted as a safe, effective alternative to surgical closure in selected cases. Cardiac perforation is rare, but usually observed only during the implant procedure. However, late cardiac perforations (days to months later) have been reported recently.
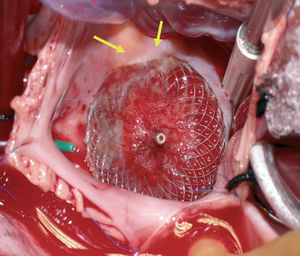
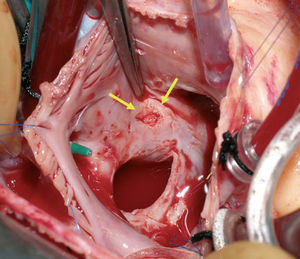
We describe a 21-year-old woman, diagnosed with an atrial septal defect consisting of ostium secundum, of diameter 22 mm, that led to considerable left-right shunt (L-R 3:1). The defect was definitively closed with an Amplatzer device of diameter 26 mm, with an uneventful postoperative period. Follow-up echocardiography showed that the device was well positioned, with no evidence of residual shunt or interference with the valve or with the outlet of large vessels. There was no pericardial effusion. At 3 weeks the patient presented syncopal symptoms. She was diagnosed with cardiac tamponade by pericardiocentesis with drawing of 150 mL of hematic fluid. Subsequent echocardiography showed the absence of perfusion, as well as correct placement of the device. Since a self-contained perforation of the atrial wall was suspected, cardiac surgery was undertaken. An ulcerated lesion was found at the edge of the device, in the cranial area of the right atrial septum, where the roofs of both atria and the aortic root meet (Figures 1 and 2). The Amplatzer device was withdrawn and the atrial defect was closed with suturing of the atrial wall wound and an autologous pericardial patch. The postoperative course was satisfactory.
Figure 1. Implanted device, seen from the right atrium, with a portion of the disk (arrows) penetrating the atrial wall.
Figure 2. Lesion following removal of the device (arrows).
Cardiac perforation related to the implant of an Amplatzer device is a rare complication that usually occurs only during placement of the device.1 However, in a data review provided by the U.S. and Canadian drug agencies, 66% of the 29 cardiac perforations reported were late (post-discharge), 24% occurred 1-6 months later, and only 1 occurred more than a year later (3 years).2 The incidence is not assessable, as the total number of implants recorded is not provided. The patients with cardiac perforation presented chest pain, dyspnea, syncope, hemodynamic collapse, or even sudden death; where surgery or autopsy was performed, the cardiac perforation was found to be in the anterosuperior wall of the atria and/or in the adjacent aorta.
A deficient anterosuperior border or the insertion of a grossly oversized device have been mentioned as predisposing factors.3 Septal aneurysm has not been associated with further complications on follow-up.4 The actual incidence of this complication must be low, since the published series do not describe late perforations. In a series of 417 patients, only one late peripheral embolization and one late sudden death were described.1 Another study assessed 151 long-term (5-9 years) cases, without observing any deaths or major complications.5
In our setting, based on data provided by the Sección de Hemodinámica (Hemodynamics Section) of the Sociedad Española de Cardiología (Spanish Society of Cardiology),6 345 cases of atrial septal defect were closed percutaneously in 2005, with an incidence of 2.9% for complications and 1.2% for death.
Although rare, the potential fatal outcome of this condition requires strict selection criteria for patients and for choosing device sizes, as well as careful postoperative monitoring of these patients, with frequent echocardiographic follow-up during the first 6 months, particularly in the first month. If there is clear evidence of this complication, then the safest approach would probably be surgical withdrawal of the device and conventional closure of the defect once other potential causes for the patient's symptoms are ruled out.