Ivabradine is an inhibitor of the If channel, the main determinant of the pacemaker function of the sinus node. The drug has been approved for the treatment of angina and heart failure. There is some evidence of its role as an inhibitor of atrial-ventricular node (AVN) conduction. The aim of the BRAKE-AF project is to assess ivabradine use for rate control in atrial fibrillation (AF).
MethodsA multicenter, randomized, parallel, open-label, noninferiority phase III clinical trial will be conducted to compare ivabradine vs digoxin in 232 patients with uncontrolled permanent AF despite beta-blockers or calcium channel blockers. The primary efficacy endpoint is the reduction in daytime heart rate measured by 24-hour Holter monitoring at 3 months. This clinical trial will be supported by an electrophysiological study of the effect of ivabradine on the action potential of the human AVN. To do this, an experimental model will be used with Chinese hamster ovarium cells transfected with the DNA encoding the expression of the t channels involved in this action potential and recording of the ionic currents with patch clamp techniques.
ResultsNew data will be obtained on the effect of ivabradine on the human AVN and its safety and efficacy in patients with permanent AF.
ConclusionsThe results of the BRAKE-AF project might allow inclusion of ivabradine within the limited arsenal of drugs currently available for rate control in AF.
Clinical trial registration: http://www.clinicaltrials.gov. Identifier: NCT03718273.
Keywords
Atrial fibrillation (AF) is the most common sustained arrhythmia, with an increasing prevalence due to population aging.1 Among the strategies available to treat the symptoms, heart rate (HR) control is essential and is the only option for patients with permanent AF. This can be achieved with drugs that modulate the various currents generated by the action potential of the atrioventricular node (AVN).2
At present, 3 groups of drugs are available to control HR: beta-blockers, nondihydropyridine calcium channel blockers, and digoxin, all of which currently have the same recommendation class (I) and level of evidence (B) in the latest European guidelines for AF.3 These guidelines state that further research is needed into drugs and strategies aimed at controlling HR in AF. Usually, beta-blockers or nondihydropyridine calcium channel blockers (diltiazem, verapamil) are the first therapeutic step because they have a more favorable efficacy and safety profile than digoxin. In fact, digoxin is associated with a number of problems, including poor HR control during exertion,4–6 a narrow therapeutic range, multiple drug interactions, and a possible increase in long-term mortality.7,8 At the present time, there are no alternative drugs to digoxin for HR control in AF except for beta-blockers or calcium channel blockers, which require pacemaker implantation and AVN ablation to control symptoms in certain cases and, consequently, leave the patient dependent on pacing.
Ivabradine is an antiarrhythmic that inhibits the depolarizing If channel, a mixed sodium/potassium inward current initially described in pacemaker cells of the sinus node,9 where it is the main determinant of its automaticity. This drug has seen major developments and has been used clinically due to its bradycardiac effect on the sinus node, and unlike beta-blockers and nondihydropyridine calcium channel blockers, it does not affect blood pressure, cardiac contractility, refractory periods, or intracardiac conduction velocity. The safety and clinical benefits of the drug have been confirmed in 3 large clinical trials. In the BEAUTIFUL10 and SIGNIFY11 studies with patients diagnosed with coronary disease, the drug reduced myocardial infarction-related hospitalizations and improved anginal symptoms, respectively. Additionally, the SHIFT12 study, conducted in patients with reduced ejection fraction, reported that ivabradine lowered the primary endpoint of mortality and hospitalization due to heart failure.
The usefulness of ivabradine as a negative chronotropic drug in patients with permanent AF has not been established in clinical practice. The aim of the BRAKE-AF project is to investigate the potential role of ivabradine within the therapeutic strategy used to control heart function in AF.
METHODSThe BRAKE-AF translational project includes 2 parallel research studies: a) a basic research study on the effect of ivabradine on the various currents involved in the genesis of the AVN action potential in a cell model, and b) a multicenter, randomized, controlled, parallel-group, open-label, noninferiority phase III clinical trial to compare ivabradine vs digoxin in patients with permanent AF and rapid ventricular response.
The BRAKE-AF project is being carried out at 10 Spanish sites (9 hospitals and the School of Medicine at the Universidad Complutense de Madrid) and is funded by the Carlos III Research Institute through an AES grant (PI17/01272). The clinical trial has been approved by the Research Ethics Committee for drugs and by the Agency of Medicines and Medical Devices. It has been registered in the ClinicalTrials.gov database under the identifier NCT03718273 and in the European Clinical Trials (EudraCT) database under No. 2018-001936-23.
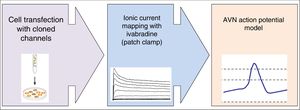
Basic research studyA diagram of the basic research study design is shown in figure 1.
- •
To determine the effects of ivabradine on the amplitude of currents generated by human AVN channels: Ca2+ inward currents through L- (ICaL) and T-type (ICaT) channels and various K+ outward currents: the transient (Ito), ultrarapid (IKur), rapid (IKr), and slow (IKs) components of the delayed rectifier current and the inward rectifier IK1 current.
- •
To determine the time- and voltage-dependent effects of ivabradine on the activation, inactivation, and reactivation processes of these currents.
- •
To include these data in a model of the AVN-generated action potential to learn how ivabradine could affect them.
Chinese hamster ovarian cells will be transfected with DNA coding for the expression of alpha and beta subunits of ionic channels cloned from human myocardial tissue involved in the AVN action potential, and cotransfected with the EBO-pcD leu 2 vector (0.5 ug), which codes for the expression of CD8 surface antigen, making it possible to identify the cells for subsequent collection and use in electrophysiological recordings. The experiments will be performed in external control solutions and in ivabradine solutions of varying concentrations.
Data collection and analysisIonic currents will be recorded using the whole-cell patch-clamp technique and following a previously described protocol.13,14 The external and micropipette solutions will be specific for each ionic current to be recorded. Capacitive current artifacts generated after applying pulses of –80 to –70mV will be recorded at 50kHz (filtered at 10kHz) to calculate cell capacitance, access resistance, and input impedance. The capacitance and series resistances will be compensated up to 80% in all experiments. Data acquisition, analysis, and pulse protocols will be controlled by the PCLAMP program (Molecular Devices). During the experiments, the membrane potential and ionic currents will be monitored continuously on a digital oscilloscope (model 5020 A, Kikusui Electronics Co).
The activation and inactivation curves of the various currents will be adjusted by a Boltzmann equation, in order to determine the possible effects of mutations on activation or inactivation voltage dependence. The activation, inactivation, and reactivation kinetics will be studied by an exponential analysis of the current recordings.
The study will analyze how the electrophysiological abnormalities observed affect the characteristics of the node action potentials. The model will be implemented with MATLAB6.5 (MAThematical LABoratory, Mathworks Inc, Natick, Massachusetts, United States) using an ode15s integration algorithm under baseline (control) conditions and at various pacing frequencies (0.5-3.0Hz) for at least 200 cycles to ensure that all parameters reach steady state. The following parameters will be recorded from the simulated action potentials: resting potential (mV), amplitude (mV), and duration (ms), recorded at 20% (APD20), 50% (APD50), and 90% (APD90) of repolarization, allowing prediction of the effects of ivabradine on the node action potential.
Statistical analysisThe results will be expressed as the mean±standard deviation. The data obtained from each group will be compared with the data from their respective controls using the Student t test. To perform between-group comparisons, ANOVA (analysis of variance) will be used, followed by a Newman-Keuls test.
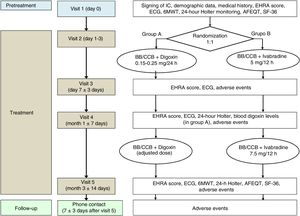
Clinical trialThe clinical study has been designed as a multicenter, randomized, controlled, parallel-group, open-label, noninferiority phase III trial (figure 2).
Flowchart of the BRAKE-AF clinical trial visits and procedures. 6MWT, 6minute walk test; AFEQT, Atrial Fibrillation Effect on QualiTy-of-life; BB, beta-blockers; CCB, calcium channel blockers (nondihydropyridine); ECG, electrocardiogram; EHRA, European Heart Rhythm Association; IC, informed consent; SF-36, The Medical Outcomes Study 36-item Short-Form Health Survey.
The trial will evaluate whether or not ivabradine dosing used as adjuvant treatment to beta-blockers or nondihydropyridine calcium channel blockers is a noninferior alternative to digoxin for reducing HR in patients with permanent AF. The following outcome measures have been defined:
- •
Primary efficacy outcome measure: Reduction in mean daytime HR recorded by Holter-electrocardiogram (ECG) at 3 months.
- •
Primary safety outcome measure: Composite of:
- -
Syncope
- -
Severe bradycardia: Sustained HR <40 bpm (for at least 10 s) or asystole> 4 s resulting in syncope or symptoms requiring therapeutic measures (intravenous chronotropic drugs) or pacing (transcutaneous or by temporary pacemaker).
- -
Serious adverse reaction resulting in hospitalization, emergency visit, or death.
- -
- •
Secondary efficacy outcome mesures:
- -
Symptoms consistent with the EHRA score assessed at 1 and 3 months.
- -
Distance covered in the 6-minute walk test at 3 months of treatment.
- -
Quality-of-life metrics analyzed in the SF-3615 and AFEQT16 questionnaires at 1 and 3 months.
- -
Mean daytime HR recorded by Holter-ECG at 1 and 3 months.
- -
Resting HR recorded by ECG at 1 and 3 months.
- -
Maximum HR recorded by Holter-ECG at 1 and 3 months.
- -
Mean HR over 24hours recorded by Holter-ECG at 1 and 3 months.
- -
HR delta (difference between maximum HR and mean HR) recorded by Holter-ECG at 1 and 3 months.
- -
HR during moderate exercise recorded by Holter-ECG during the 6-minute walk test at 3 months of treatment.
- -
- •
Secondary safety outcome measures. Composite of:
- -
Nonserious bradycardia: Sustained HR <40 bpm (for at least 10 s) or asystole> 4 s with no clinical repercussions or resulting in mild (nonsyncopal) symptoms not requiring therapeutic measures or pacing.
- -
Any serious adverse reaction to the study drugs.
- -
Voluntary discontinuation of the drug by the patient.
- -
Hospitalizations, emergency department visits, and cardiovascular mortality during treatment with the study drugs.
- -
Patients will be selected if they have permanent AF and poor HR control in the absence of secondary factors causing the condition, despite beta-blocker or nondihydropyridine calcium channel blocker therapy. The selection criteria are summarized in table 1.
BRAKE-AF clinical trial population. Selection criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age ≥ 18 y | Previous treatmenta or patients with a known contraindication to ivabradine or digoxin or to any excipient of either drug |
| Permanent AF at randomization, with no prospect of cardioversion, antiarrhythmic treatment with group I or III drugs, or pulmonary vein ablation | Paroxysmal or intermittent complete AV block in patients without a pacemaker |
| Symptoms attributable to AF and associated with the presence of at least 1 of the following criteria of inadequate HR control: resting HR> 110 bpm or resting HR of 80-110 bpm and at least 1 of the following criteria:• HR during moderate exerciseb> 130 bpm (stress test or Holter-ECG)• Mean daytime HRc ≥ 80 bpm (Holter-ECG) | Decompensated heart failure, requiring intravenous diuretics or inotropics in the week prior to randomization or in NYHA functional class IV or on the heart transplant waiting list |
| Beta-blocker or nondihydropyridine calcium channel blocker therapy at the maximum dose recommended for or tolerated by the patient | Acute pericarditis, acute myocarditis, constrictive pericarditis, hypertrophic obstructive cardiomyopathy |
| Blood work in the 6 mo prior to inclusion including complete blood count, thyroid hormone, and creatinine to rule out secondary causes of poor HR control | Severe hypotension (< 90/50 mmHg) |
| Transthoracic echocardiogram to rule out serious valve disease, hypertrophic cardiomyopathy, etc. Blood work performed in the year before study enrollment will be considered acceptable, provided that the patient's clinical status has been stable during this period | Kidney failure (CrCl <30 mL/kg/min) or in a hemodialysis program |
| Serious liver failure | |
| Major surgery (including cardiac) in the month prior to randomization | |
| Valve disease requiring surgical or percutaneous repair | |
| Concomitant treatment with potent inhibitors of cytochrome P-450 isoenzyme 3A4d | |
| Serious concomitant disease with life expectancy <1 y | |
| Acute myocardial infarction, unstable angina (during its acute phase), or recent stroke (past 4 wk) | |
| Congenital long QT syndrome or patients treated with medications that prolong this interval | |
| Woman of childbearing age (< 50 y, unless submitting a gynecologic report certifying menopause) and women who are breastfeeding | |
| Impossibility to attend the visits scheduled in the protocol | |
| Participation in a clinical trial in the previous 6 mo | |
| Be able to voluntarily give informed consent | Medical causes that explain poor HR control: fever, anemia, hyperthyroidism, pheochromocytoma, etc. |
AF, atrial fibrillation; AV, atrioventricular; CrCl, creatinine clearance; ECG, electrocardiogram; HR, heart rate; NYHA, New York Heart Association.
Previous digoxin treatment is considered continuous treatment with this drug by the same administration route as the comparator experimental drug (oral digoxin), allowing the patient to acquire a drug concentration able to provide the desired therapeutic effect (HR control).
Corresponds to a workload of about 3 and 6 METS (metabolic equivalents) equivalent to the workload performed during the first 2 Bruce protocol stages of a stress test. Some examples of this type of exercise are: walking at a leisurely pace, doing household chores, dancing, gardening, carrying loads weighing less than 20kg, sexual activity, washing a car, etc.
Trial patients will be randomized to 1 of 2 groups at a ratio of 1:1. In the digoxin group, the starting dose will be selected according to the presence of 3 factors: age ≥80 years, weight ≤60kg, and creatinine clearance <60mL/min as described below: 0.25mg/24h (no factors), 0.15mg/24h (1 factor), or 0.10mg/24h (2 or 3 factors). The 0.25mg tablet formulation will be used for the 0.25mg/24h dose, and the pediatric solution (0.05mg/mL) will be used for all other doses. The doses will be adjusted as needed during follow-up to maintain digoxin concentrations within the therapeutic range (0.5-2 ng/mL). In the ivabradine group, a dose of 5mg/12h will be administered for 1 month. Afterwards, if the dose is well tolerated and Holter monitoring shows no significant bradycardia (HR <40 bpm maintained for> 10 s or asystole> 4 s), it will be increased to a maintenance dose of 7.5mg/12h. Patients aged 75 years or older randomized to ivabradine will be administered a starting dose of 2.5mg/12h with the possibility of raising the dose to 5mg/12h in the following week according to tolerance and in the absence of bradycardia (HR <60 bpm) in the resting ECG.
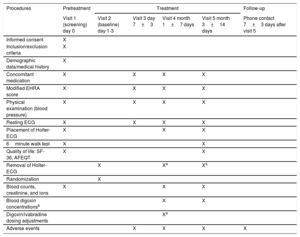
VisitsThe clinical trial will be conducted over 5 face-to-face visits: 1 for screening (to confirm that the patient meets all the selection criteria) and to sign the informed consent and 4 for treatment (randomization and follow-up at 1 week, 1 month, and 3 months). Additionally, a follow-up telephone visit will be performed 1 week after discontinuation of the study medication. The procedures performed at the various clinical trial visits are listed in table 2.
BRAKE-AF trial visit and procedure schedule:
| Procedures | Pretreatment | Treatment | Follow-up | |||
|---|---|---|---|---|---|---|
| Visit 1 (screening) day 0 | Visit 2 (baseline) day 1-3 | Visit 3 day 7±3 | Visit 4 month 1±7 days | Visit 5 month 3±14 days | Phone contact 7±3 days after visit 5 | |
| Informed consent | X | |||||
| Inclusion/exclusion criteria | X | |||||
| Demographic data/medical history | X | |||||
| Concomitant medication | X | X | X | X | ||
| Modified EHRA score | X | X | X | X | ||
| Physical examination (blood pressure) | X | X | X | X | ||
| Resting ECG | X | X | X | X | ||
| Placement of Holter-ECG | X | X | X | |||
| 6minute walk test | X | X | ||||
| Quality of life: SF-36, AFEQT | X | X | ||||
| Removal of Holter-ECG | X | Xa | Xa | |||
| Randomization | X | |||||
| Blood counts, creatinine, and ions | X | X | X | |||
| Blood digoxin concentrationsb | X | X | ||||
| Digoxin/ivabradine dosing adjustments | Xa | |||||
| Adverse events | X | X | X | X | ||
AFEQT, Atrial Fibrillation Effect on QualiTy-of-life; ECG, electrocardiogram; EHRA, European Heart Rhythm Association; SF-36, The Medical Outcomes Study 36-item Short-Form Health Survey.
The reference for this trial is a study by Farshi et al.6 that investigated mean heart rates using various therapeutic regimens. A reduction of 12 bpm in mean daytime HR was observed when digoxin was added in patients receiving baseline treatment with diltiazem; the same reduction was also seen in patients receiving baseline treatment with atenolol (with standard deviations of 17.4 and 10.3 bpm, respectively).
The total number of patients required for this noninferiority study will be 232 participants (116 patients in each treatment arm). This estimate was based on the following assumptions: 1-tailed hypothesis at an α level of 5%, power of 80%, and a specified noninferiority limit of difference in HR reduction between ivabradine and digoxin of –6 bpm (50% reduction in previously observed mean daytime HR), standard deviation of daytime HRs of 17.4 bpm (largest standard deviation found),5 and expected losses to follow-up of 10% of patients.
Losses to follow-upTo prevent losses during follow-up, telephone contact will be made before each visit. An analysis will be performed to determine the reason for missing data related to patient follow-up and clinical progress. In the case of losses to follow-up, variables will be replaced with the measurement closest in time if the number of losses does not account for more than 40% of missing values.
RandomizationRandomization will be stratified by site and by baseline treatment (beta-blocker or calcium channel blocker) and will be performed in a centralized manner through a website that will keep the sequence hidden.
Patient withdrawal criteriaPatients will be withdrawn at their own request, if there is significant noncompliance with the treatment or visit schedule, if they require a prohibited concomitant treatment (eg, class IC or III antiarrhythmics), if they undergo AVN ablation, if they experience a serious or clinically relevant adverse reaction deemed by the investigator to require withdrawal, or if they die.
End-of-study criteriaThe study will be ended if there are unexpected risks considered unacceptable for patients, if an acceptable number of patients cannot be enrolled, if there is inadequate compliance with protocol requirements, or if there is any discontinuation or disruption in the supply of study drugs by the pharmaceutical companies marketing them.
Statistical analysisIf the data exhibit a normal distribution (Kolmogorov-Smirnov test), the efficacy outcome measures will be evaluated by the Student t test for mean differences: distance walked, quality-of-life metrics, and HR in the various assessments. Conversely, the Mann-Whitney U-test will be used if the distribution is not normal. Symptoms according to the modified EHRA score will be evaluated using the Cochran-Mantel-Haenszel test. Outcome measures indicating a change between 2 measurements over time may be evaluated by analyzing the change in score or, complementarily, by a covariance analysis of the subsequent score. Between-treatment safety outcome measures will be compared using the chi-square or Fisher exact test. The significance level will be .05 (2-tailed α error of 5%) for all comparisons, except for the primary efficacy outcome measure of the study, in which case a 2-tailed significance level of .10 will be used. The efficacy analysis will be performed using the intention-to-treat population. All patients who received at least 1 dose of the assigned treatment will be included in the safety analysis. The study will consider assessing the relationship between the treatment and the adjusted efficacy outcome measure in a multivariate model with age, sex, and concomitant baseline treatment (beta-blockers or calcium channel blockers).
DISCUSSIONBRAKE-AF is a translational research project aiming to evaluate the potential role of ivabradine in controlling heart function during AF through a cellular-level study to analyze how ivabradine modifies the action potential of human AVN and, in parallel, through a clinical trial in patients with permanent AF to study the bradycardiac effect of the drug. The internal validity of the clinical trial is determined by its randomized design, comprehensive HR monitoring performed in all patients, and intention-to-treat analysis. External validity is based on the multicenter design and on the fact that the study population closely represents patients with poorly controlled permanent AF seen in clinical practice.
Although it is true that the If current is the main determinant of sinus node automaticity, this current is not found only in the sinus node. The first evidence of this fact was observed in anesthetized dogs, in which If inhibition with zatebradine reduced the AVN's intrinsic firing rate and the increase in AVN rate in response to sympathetic pacing.17 It later became obvious that an If inward current was present during the hyperpolarization phase (–60 to –90mV) in subsidiary pacemaker cells of the AVN.18 More recently, it has been shown by immunohistochemistry that HCN channels responsible for the If current are present in both the compact AVN and its posterior nodal extensions.19 In patients without structural heart disease, it has also been observed that intravenous administration of zatebradine, an If current inhibitor, produced significant elongation of the atrial-His (A-H) interval and prolongation of the AVN effective refractory period, but did not modify intra-atrial or intraventricular conduction.20 In another experimental study conducted in an AF model in guinea pigs, ivabradine prolonged A-H intervals in a frequency-dependent manner and lowered ventricular rate during AF.21 However, no changes were observed in the QT and H-V intervals, the dominant frequency of AF, blood pressure, or cardiac contractility.
There is a paucity of evidence on the clinical use of ivabradine in patients with AF, although the data are promising. In a series of 6 patients with rapid AF treated with beta-blockers, ivabradine improved control of the mean ventricular response and functional capacity in 4 of them.22 Last, a randomized clinical trial with 32 patients who had nonparoxysmal AF showed that ivabradine reduces mean and maximum HR without producing adverse effects.23 In the same line, it has been published that ivabradine used for this indication under compassionate use may be an effective and safe alternative to AVN ablation if there is treatment failure with drugs that depress AVN conduction.24,25 More recently, it has been reported that ivabradine was effective in treating congenital junctional ectopic tachycardia in 3 pediatric patients.26
Ivabradine is already widely used in clinical medicine, and the possibility to demonstrate its usefulness in controlling HR in AF patients may provide an innovative and relevant option, as the compound has a proven favorable safety profile in patients with structural heart disease. Additionally, the absence of effects on cardiac contractility and the vascular system makes it particularly attractive as an antiarrhythmic.
LimitationsThe experimental transfected-cell design requires the use of mathematical models to estimate the effect of ivabradine on the AVN action potential, as it is not possible to validate this effect in actual node tissue.
This clinical trial is open-label for both the patient and the investigator, mainly because the active comparator (digoxin) produces obvious repolarization abnormalities in the ECG and has a narrow therapeutic range and, therefore, occasionally requires dose titration and monitoring of drug concentrations. Although bias may arise in subjective metrics such as symptoms or quality of life, it is unlikely to significantly affect the primary outcome measure of the study (mean daytime HR recorded on Holter monitoring). This limitation will be mitigated by a blind evaluation of the study outcome measures, following disaggregation of data on the treatment received in centralized analyses of the Holter recordings and the between-group comparison.
CONCLUSIONSBRAKE-AF is a multicenter research project aiming to evaluate the efficacy and safety of ivabradine in controlling HR in AF vs digoxin in patients with failure to first-line drugs. It is based on research on the mechanism of action of ivabradine at the cellular level intended to investigate the effect of the drug on the AVN action potential.
This project has the potential to add ivabradine to the limited number of drugs currently available for HR control in AF.
FUNDINGFunding for this study has been provided by a grant awarded through a public competitive call by the Ministry of Economy and Competitiveness of Spain through the ISCIII (Carlos III Health Institute). Dossier No. PI17/01272. It has the support of the Plataforma Española de Investigación Clínica y Ensayos Clínicos, SCReN (Spanish Clinical Research Network), funded by the ISCIII-Subdirección General de Evaluación y Fomento de la Investigación under project PT13/0002/0012, PT17/0017/0011, integrated with the Plan Estatal de I+D+I 2013-2016 and cofunded by the European Regional Development Fund.
CONFLICTS OF INTERESTM.A. Arias is Associate Editor of Revista Española de Cardiología.
- –
HR control is essential for AF treatment, although the therapeutic armamentarium currently available is limited and has drawbacks.
- –
Little effort has been made to understand new drugs that could be used as negative chronotropic drugs in AF.
- –
Ivabradine is a bradycardiac drug shown to have a good safety profile in coronary patients and in patients with ventricular dysfunction.
- –
This will be the first study to investigate the effect of ivabradine on the action potential of human AVN in depressing AVN conduction.
- –
A clinical trial will be undertaken to investigate the use of ivabradine in clinical practice as a negative chronotropic drug in patients with permanent AF.
- –
Evidence for ivabradine noninferiority compared with digoxin in controlling HR in AF, in terms of both safety and efficacy, may broaden the range of drugs available in this clinical context.
The authors would like to thank Dr Héctor Bueno Zamora (Head of the Translational Multidisciplinary Research Group of the National Cardiovascular Research Center) for his ongoing support and enthusiasm since the earliest phases of this project. We would especially like to thank Rosa Vega Viaña, project manager at the Clinical Research and Scientific Support Unit of SCReN at Hospital 12 de Octubre in Madrid, for her magnificent work and untiring devotion to the study.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.09.004