Fractional flow reserve or instantaneous wave-free ratio has become a standard criterion for revascularization. We sought to evaluate the association between intravascular ultrasound (IVUS) or optical coherence tomography (OCT)-derived quantitative plaque characteristics and the severity of physiologic stenosis.
MethodsA total of 365 stenoses from 330 patients were evaluated. The association between IVUS or OCT-derived parameters and resting physiologic indices (instantaneous wave-free ratio, resting full-cycle ratio, and diastolic pressure ratio) and fractional flow reserve were explored.
ResultsAmong the total number of lesions, 50.7% and 58.1% showed an instantaneous wave-free ratio ≤ 0.89 and fractional flow reserve ≤ 0.80, respectively. IVUS or OCT-derived parameters showed significant correlations with resting physiologic indices (P values <.005). The best cutoff values of IVUS minimum lumen area (MLA), plaque burden, OCT-MLA, and OCT-area stenosis to predict functional significance were the same (IVUS-MLA: 3.4 mm2, plaque burden: 72.0%, OCT-MLA: 2.0 mm2, OCT-area stenosis: 68.0%) for all resting physiologic indices (instantaneous wave-free ratio, resting full-cycle ratio, and diastolic pressure ratio). The best cutoff values for fractional flow reserve were an IVUS-MLA of 3.8 mm2, plaque burden of 70.0%, OCT-MLA of 2.3 mm2, and OCT-area stenosis of 65.0%. Regardless of IVUS or OCT-derived parameters, the overall diagnostic accuracies of the parameters were lower than 70% and discrimination indices were less than 0.75 for resting physiologic indices or fractional flow reserve.
ConclusionsThe resting physiologic indices showed an identical relationship with IVUS or OCT-defined quantitative plaque characteristics. The diagnostic accuracy and discrimination ability of anatomical parameters were modest in predicting functional significance defined by resting and hyperemic invasive physiologic indices.
This trial is registered at ClinicalTrials.gov (Identifier: NCT03795714).
Keywords
Given the inherent limitations of coronary angiography to depict the presence of functionally significant epicardial coronary stenosis and the discrepancy between angiographic stenosis severity and the presence of myocardial ischemia,1,2 invasive physiologic indices such as fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) have become a standard method to guide clinical decision-making for revascularization.3 Intravascular ultrasound (IVUS) or optical coherence tomography (OCT) are intracoronary imaging methods able to provide more accurate assessment of anatomical plaque characteristics. Several previous studies have explored the diagnostic performance of IVUS- or OCT-defined quantitative parameters in predicting functional significance defined by FFR,4–18 and have shown only moderate diagnostic accuracy; the best cutoff values of IVUS or OCT-derived quantitative parameters varied according to the patient population, interrogated vessels, and the location of target lesions. Nevertheless, the adoption rate of FFR-guided decision-making is still low and intravascular image-guided decision-making is used in a substantial proportion of patients.19
Since the development of iFR, several resting pressure-derived physiologic indices, such as the resting full-cycle ratio (RFR) or diastolic pressure ratio (dPR), have been introduced in daily practice. A recent study demonstrated identical diagnostic performance20,21 and prognostic implications among iFR, RFR, and dPR.22 As these resting pressure-derived indices are easy to use during daily practice, it is expected that “ease of use” will increase the adoption rate of physiologic interrogation. Therefore, understanding the association between available physiologic indices and intravascular imaging-derived anatomic plaque characteristics might be important in clinical decision-making for patients with coronary artery disease.
We sought to evaluate the association between IVUS or OCT-derived quantitative plaque characteristics and the severity of physiologic stenosis assessed by resting and hyperemic physiologic indices (iFR, RFR, dPR, and FFR).
MethodsStudy design and patient populationConsecutive patients who underwent clinically-indicated invasive coronary angiography and who were also evaluated by invasive physiologic indices and IVUS or OCT for at least 1 coronary artery were enrolled from 2 university hospitals (Seoul National University Hospital and Samsung Medical Center) between May 2009 and November 2018. We excluded patients with hemodynamic instability, left ventricular dysfunction, or a culprit vessel of acute coronary syndrome. A part of the current study population was included in another published study.1,2,23,24 There were no mandated indications for intravascular imaging and it was performed on the basis of clinical criteria at the operators’ discretion. The specific study protocol of the current analysis was approved by the Institutional Review Board or Ethics Committee at each participating center and the study protocol was in accordance with the Declaration of Helsinki (clinicaltrials.gov identifier: NCT03795714).
Angiographic analysis and quantitative coronary angiographyCoronary angiography was performed with standard techniques. Angiographic views were obtained following the administration of intracoronary nitrate (100 or 200μg). All angiograms were analyzed at a core laboratory in a blinded fashion. Quantitative coronary angiography was performed in optimal projections with validated software (CAAS II, Pie Medical Imaging, Maastricht, The Netherlands). Percent diameter stenosis, minimum lumen diameter, reference vessel size, and lesion length were measured. The SYNTAX score was measured as previously described.25
Coronary physiologic measurementsAll coronary physiologic measurements were obtained after diagnostic angiography as previously described.2,22,23 Briefly, a 5- to 7-Fr guide catheter was used to engage the coronary artery. The pressure-temperature sensor guidewire (Abbott Vascular, Santa Clara, CA, USA) was zeroed and equalized to aortic pressure, and was then positioned at the distal segment of a target vessel. Intracoronary nitrate (100 or 200μg) was administered before each set of physiologic measurements. Resting Pd/Pa was calculated as the ratio of mean distal coronary artery pressure to mean aortic pressure in resting state. The iFR was calculated using automated algorithms acting over the wave-free period over a minimum of 5 beats as previously described.2,26,27 Briefly, resting pressure tracings were analyzed by a dedicated MatLab script using an ECG-independent algorithm.28 Continuous infusion of adenosine (140μg/kg/min) or nicorandil (2mg bolus) was used to induce hyperemia.29,30 Hyperemic proximal aortic pressure (Pa) and distal arterial pressure (Pd) were obtained, and FFR was calculated as the lowest average of 3 consecutive beats during adenosine infusion or after nicorandil administration. After measurements, the pressure wire was pulled back to the guide catheter and the presence of pressure drift was checked. All pressure readings were collected and validated at the core laboratory in a blinded fashion.
RFR was calculated from each individual waveform using a fully automated offline software algorithm (Abbott Vascular, Santa Clara, CA, USA) following standardization of the pressure sampling rate to 100Hz, as previously described.20 Briefly, a minimum of 5 consecutive heart cycles were needed to determine the RFR. To eliminate signal artifacts inherent to subcycle measurement, a low-pass filter was applied to the phasic Pd/Pa. RFR was defined as the point at which the ratio of Pd and Pa was lowest during the entire cardiac cycle.20 dPR was also calculated from each individual waveform as an average Pd/Pa over the entire period of diastole as previously described.21 The calculation of RFR and dPR from pressure tracing data was performed at the core laboratory in a blinded fashion (Samsung Medical Center). Lesions with ≤ 0.89,22,31,32 were considered functionally significant for iFR, RFR, and dPR, and ≤ 0.80 for FFR.
Intravascular ultrasound or optical coherence tomography acquisition and analysisIVUS was performed in a standard fashion using an automated motorized pullback system (0.5mm/seconds) with commercially available imaging catheters (OptiCross, Boston Scientific, Minneapolis, MN, USA) after intracoronary administration of 200μg of nitroglycerin. Images were digitally stored for offline analysis and were analyzed using commercially available software (Echoplaque 4.0, INDEC Medical System, Santa Clara, CA, USA) at a core laboratory (Seoul National University Hospital) in a blinded fashion. Evaluation of 2- and 3-dimensional lesion morphology and other measurements of IVUS images were performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies.33 Measured quantitative IVUS parameters were external elastic membrane (EEM) area, lumen cross-sectional area (CSA), minimum lumen area (MLA), and plaque plus media (P+M) area. Plaque burden (PB) was calculated as: [(P+M CSA)/(EEM CSA)] x100.
OCT images were obtained using a commercially available catheter (Dragon Fly, Abbott Vascular, Santa Clara, CA, USA). All OCT data were digitally stored and transferred to a core laboratory (Samsung Medical Center) where they were analyzed by an independent investigator in a blinded fashion. OCT measurements were performed using the proprietary software for offline analysis (LightLab Imaging). The lumen area was measured at the cross section with the smallest lumen area and the reference segment, which was defined as the frame with the largest lumen within 10mm proximal or distal to the MLA before any side branch. OCT-derived percent area stenosis (AS) was calculated as: ([reference lumen area – MLA]/reference lumen area) x 100. Stenosis length was defined as the region around the MLA where the lumen area was <50% of the reference lumen area. If there were multiple stenoses in the target vessel, the stenosis with the smallest MLA was selected as a representative lesion and used in the current analysis. The selection of imaging modality was based on the operator's discretion. No patient underwent both IVUS and OCT.
Statistical analysisCategorical variables are presented as numbers and relative frequencies (percentages) and continuous variables as means and standard deviations, whose distribution was checked by the Kolmogorov-Smirnov test. Data were analyzed on a per-patient basis for clinical characteristics and on a per-lesion basis for the other analyses. Linear regression analysis was used to estimate the correlation coefficient between quantitative variables. The association between IVUS or OCT-derived quantitative parameters and invasive physiologic indices were plotted using the LOWESS (locally weighted scatterplot smoothing) regression line. For per-lesion analyses, a generalized estimating equation was used to adjust intrasubject variability among vessels from the same patient. Estimated means and 95% confidence intervals are presented as summary statistics. No post hoc adjustment was performed.
Diagnostic performances of IVUS or OCT-derived quantitative parameters to predict functional significance defined by invasive physiologic indices are presented as sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, and diagnostic accuracy. Discriminatory function was evaluated using the area under the curve in receiver operating curve analysis, and the area under the curve was compared with the DeLong method. All probability values were 2-sided and P values <.05 were considered statistically significant. The SPSS version 18.0 (SPSS Inc, Chicago, Illinois, United States) and R 3.5.2 (R Corporation, United States) statistical packages were used for statistical analyses.
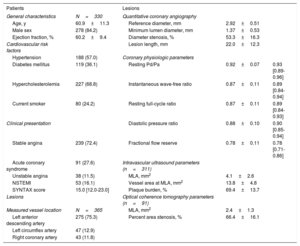
ResultsCharacteristics of patients and lesionsTable 1 shows the characteristics of the study population and target lesions. Most patients presented with stable angina (72.4%) and an intermediate stenosis with mean angiographic % DS of 53.3%±16.3% (median: 54.9%, Q1-Q3: 43.6%-65.5%), and mean FFR of 0.78±0.11 (median: 0.78, Q1-Q3: 0.71-0.86). In 311 lesions evaluated by IVUS, mean MLA was 4.1±2.6mm2 and PB was 69.4%±13.7%. In 91 lesions evaluated by OCT, mean MLA was 2.4±1.3mm2 and AS was 66.4%±16.1% (table 1).
General characteristics of patients and lesions
| Patients | Lesions | |||
|---|---|---|---|---|
| General characteristics | N=330 | Quantitative coronary angiography | ||
| Age, y | 60.9±11.3 | Reference diameter, mm | 2.92±0.51 | |
| Male sex | 278 (84.2) | Minimum lumen diameter, mm | 1.37±0.53 | |
| Ejection fraction, % | 60.2±9.4 | Diameter stenosis, % | 53.3±16.3 | |
| Cardiovascular risk factors | Lesion length, mm | 22.0±12.3 | ||
| Hypertension | 188 (57.0) | Coronary physiologic parameters | ||
| Diabetes mellitus | 119 (36.1) | Resting Pd/Pa | 0.92±0.07 | 0.93 [0.89-0.96] |
| Hypercholesterolemia | 227 (68.8) | Instantaneous wave-free ratio | 0.87±0.11 | 0.89 [0.84-0.94] |
| Current smoker | 80 (24.2) | Resting full-cycle ratio | 0.87±0.11 | 0.89 [0.84-0.93] |
| Clinical presentation | Diastolic pressure ratio | 0.88±0.10 | 0.90 [0.85-0.94] | |
| Stable angina | 239 (72.4) | Fractional flow reserve | 0.78±0.11 | 0.78 [0.71-0.86] |
| Acute coronary syndrome | 91 (27.6) | Intravascular ultrasound parameters (n=311) | ||
| Unstable angina | 38 (11.5) | MLA, mm2 | 4.1±2.6 | |
| NSTEMI | 53 (16.1) | Vessel area at MLA, mm2 | 13.8±4.8 | |
| SYNTAX score | 15.0 [12.0-23.0] | Plaque burden, % | 69.4±13.7 | |
| Lesions | Optical coherence tomography parameters (n=91) | |||
| Measured vessel location | N=365 | MLA, mm2 | 2.4±1.3 | |
| Left anterior descending artery | 275 (75.3) | Percent area stenosis, % | 66.4±16.1 | |
| Left circumflex artery | 47 (12.9) | |||
| Right coronary artery | 43 (11.8) | |||
MLA, minimum lumen area; NSTEMI, non-ST-elevation myocardial infarction; Pd/Pa, distal to aortic coronary pressure; SYNTAX, Synergy between percutaneous coronary intervention with Taxus and Cardiac Surgery score.
Values are expressed as mean±standard deviation, median [interquartile ranges, 25th-75th], or No. (%).
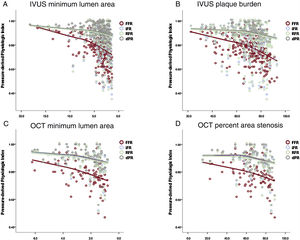
All the anatomical parameters for lesion severity showed significant correlations with invasive physiologic indices (figure 1). RFR, dPR and iFR showed a similar correlation coefficient with IVUS-MLA (R=0.253, 0.245, and 0.244, respectively, all P values <.001), PB (R=−0.243, −0.237, −0.236, respectively, all P values <.001), OCT-MLA (R=0.358, 0.346, and 0.351, respectively, all P values <.001), and OCT-AS (R=0.300, −0.295, and −0.303, respectively, all P values <.001). FFR showed a stronger correlation with the above parameters than resting physiologic indices (IVUS-MLA, R=0.410; PB, R=−0.423; OCT-MLA, R=0.434; OCT-AS, R=-0.379; all P values <.001; all P values for comparison with resting indices <.001). In addition, there was significant correlation between IVUS lesion length and all the physiologic indices (R=−0.233, −0.220, −0.229, −0.260 for iFR, RFR, dPR, and FFR). In subgroup analysis of lesion length ≥ 20mm, reference vessel diameter ≥ 3.0mm, or LAD as target vessel, the correlation between IVUS of OCT parameters and FFR was consistently higher than that with resting indices (table 1 of the supplementary data).
Association between pressure-derived indices and IVUS or OCT-derived parameters. The scatter plots show the association of IVUS minimum lumen area (A), IVUS plaque burden (B), OCT minimum lumen area (C), and OCT percent area stenosis (D) with invasive physiologic indices (iFR, RFR, dPR, and FFR). All parameters showed a significant correlation with invasive physiologic indices. dPR, diastolic pressure ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; OCT, optical coherence tomography; RFR, resting full-cycle ratio.
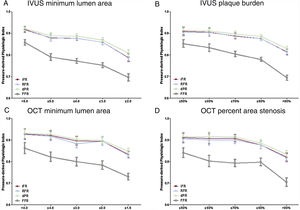
Figure 2 and table 2 show the value of invasive physiologic indices according to different stenosis severity assessed by IVUS-MLA, PB, OCT-MLA, and AS. All resting physiologic indices significantly decreased as the IVUS-MLA or OCT-MLA decreased, and IVUS-PB or OCT-AS increased (all P values <.010). Regardless of the anatomical stenosis severity indices, significant transition points of iFR, RFR, and dPR were identical for all the criteria (IVUS-MLA ≤ 4.0mm2, IVUS-PB> 80%, OCT-MLA ≤ 1.5mm2, OCT-AS> 80%) (table 2).
Changes of invasive physiologic indices according to IVUS or OCT-derived parameters. Changes in resting and hyperemic invasive physiologic indices according to IVUS minimum lumen area (A), IVUS plaque burden (B), OCT minimum lumen area (C), and OCT percent area stenosis (D). dPR, diastolic pressure ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; OCT, optical coherence tomography; RFR, resting full-cycle ratio.
Comparison of resting indices according to different angiographic and hemodynamic stenosis severity
| IVUS minimum lumen area, mm2 (N=311) | Pb | |||||
|---|---|---|---|---|---|---|
| > 5.0mm2 | 4.0-5.0mm2 | 3.0-4.0mm2 | 2.0-3.0mm2 | ≤ 2.0mm2 | ||
| No. | 67 | 45 | 88 | 76 | 35 | |
| Resting Pd/Pa | 0.95±0.01 | 0.92±0.01a | 0.92±0.01 | 0.91±0.01 | 0.86±0.02 | <.001 |
| iFR | 0.92±0.01 | 0.88±0.02a | 0.88±0.01 | 0.86±0.01 | 0.79±0.02 | <.001 |
| RFR | 0.92±0.01 | 0.88±0.02a | 0.88±0.01 | 0.86±0.01 | 0.79±0.02 | <.001 |
| dPR | 0.92±0.01 | 0.89±0.01a | 0.89±0.01 | 0.87±0.01 | 0.81±0.02 | <.001 |
| FFR | 0.86±0.01 | 0.79±0.02a | 0.77±0.01 | 0.75±0.01 | 0.70±0.02 | <.001 |
| IVUS plaque burden, % (N=311) | Pb | |||||
|---|---|---|---|---|---|---|
| ≤ 50% | 50%-60% | 60%-70% | 70%-80% | > 80% | ||
| No. | 32 | 34 | 62 | 117 | 66 | |
| Resting Pd/Pa | 0.95±0.01 | 0.94±0.01 | 0.93±0.01 | 0.93±0.01 | 0.89±0.01a | <.001 |
| iFR | 0.91±0.01 | 0.91±0.02 | 0.89±0.01 | 0.88±0.01 | 0.81±0.02a | <.001 |
| RFR | 0.91±0.01 | 0.90±0.01 | 0.89±0.01 | 0.88±0.01 | 0.81±0.02a | <.001 |
| dPR | 0.91±0.01 | 0.91±0.01 | 0.90±0.01 | 0.88±0.01 | 0.83±0.02a | <.001 |
| FFR | 0.85±0.02 | 0.83±0.02 | 0.80±0.01 | 0.78±0.01 | 0.69±0.01a | <.001 |
| OCT minimum lumen area, mm2 (N=91) | Pb | |||||
|---|---|---|---|---|---|---|
| > 4.0 mm2 | 3.0-4.0 mm2 | 2.0-3.0 mm2 | 1.5-2.0 mm2 | ≤ 1.5 mm2 | ||
| No. | 11 | 15 | 17 | 18 | 30 | |
| Resting Pd/Pa | 0.95±0.01 | 0.94±0.01 | 0.93±0.01 | 0.93±0.01 | 0.90±0.01a | .004 |
| iFR | 0.93±0.01 | 0.92±0.02 | 0.90±0.02 | 0.89±0.01 | 0.83±0.02a | .001 |
| RFR | 0.93±0.01 | 0.92±0.01 | 0.88±0.02 | 0.89±0.02 | 0.83±0.02a | .001 |
| dPR | 0.93±0.01 | 0.93±0.01 | 0.90±0.02 | 0.90±0.01 | 0.85±0.02a | .002 |
| FFR | 0.86±0.01 | 0.82±0.01 | 0.80±0.03 | 0.78±0.02 | 0.73±0.02a | <.001 |
| OCT percent area stenosis, % (N=91) | Pb | |||||
|---|---|---|---|---|---|---|
| ≤ 50% | 50%-60% | 60%-70% | 70%-80% | > 80% | ||
| No. | 14 | 11 | 25 | 22 | 19 | |
| Resting Pd/Pa | 0.94±0.01 | 0.93±0.02 | 0.93±0.01 | 0.93±0.01 | 0.88±0.01a | .012 |
| iFR | 0.91±0.02 | 0.91±0.02 | 0.91±0.02 | 0.88±0.02 | 0.82±0.02a | .003 |
| RFR | 0.91±0.02 | 0.90±0.02 | 0.90±0.02 | 0.88±0.02 | 0.82±0.02a | .005 |
| dPR | 0.92±0.02 | 0.92±0.02 | 0.91±0.01 | 0.89±0.02 | 0.83±0.02a | .003 |
| FFR | 0.84±0.01 | 0.80±0.03 | 0.79±0.02 | 0.80±0.01 | 0.70±0.02a | <.001 |
dPR, diastolic pressure ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; OCT, optical coherence tomography; Pd/Pa, distal to aortic coronary pressure; RFR, resting full-cycle ratio.
Values are expressed as mean±95% standard error.
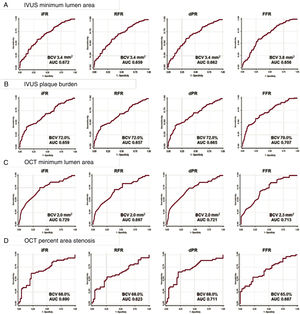
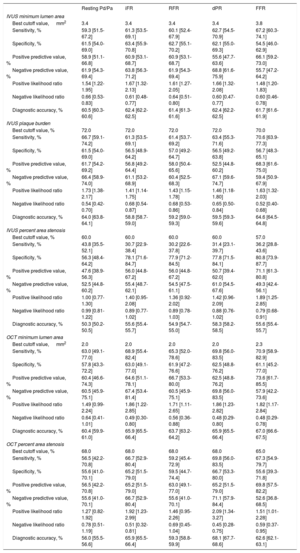
Figure 3 and table 3 show the best cutoff value of IVUS or OCT-derived parameters to predict functionally significant stenosis defined by iFR, RFR, dPR, or FFR and their diagnostic performances. The best cutoff value of IVUS-MLA, IVUS-PB, IVUS-AS, OCT-MLA, and OCT-AS were the same among resting physiologic indices (IVUS-MLA 3.4mm2, IVUS-PB 72.0%, IVUS-AS 60.0%, OCT-MLA 2.0mm2, and OCT-AS 68.0%, respectively). The best cutoff values of IVUS or OCT-derived parameters for FFR were identified in cases of less stenosis severity (IVUS-MLA 3.8mm2, IVUS-PB 70.0%, IVUS-AS 57.0%, OCT-MLA 2.3mm2, and OCT-AS 65.0%, respectively) (figure 3).
Diagnostic performance of anatomical parameters for functionally significant lesions defined by invasive physiologic indices. Best cutoff value (BCV) and area under the curve (AUC) of IVUS minimum lumen area (A), IVUS plaque burden (B), OCT minimum lumen area (C), and OCT percent area stenosis (D) for iFR, RFR, dPR, and FFR, respectively. AUC, area under the curve; BCV, best cutoff value; dPR, diastolic pressure ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; OCT, optical coherence tomography; RFR, resting full-cycle ratio.
Diagnostic performance of anatomical parameters for functionally significant lesions defined by invasive physiologic indices
| Resting Pd/Pa | iFR | RFR | dPR | FFR | |
|---|---|---|---|---|---|
| IVUS minimum lumen area | |||||
| Best cutoff value, mm2 | 3.4 | 3.4 | 3.4 | 3.4 | 3.8 |
| Sensitivity, % | 59.3 [51.5-67.2] | 61.3 [53.5-69.1] | 60.1 [52.4-67.9] | 62.7 [54.5-70.9] | 67.2 [60.3-74.1] |
| Specificity, % | 61.5 [54.0-69.0] | 63.4 [55.9-70.8] | 62.7 [55.1-70.2] | 62.1 [55.0-69.3] | 54.5 [46.0-62.9] |
| Positive predictive value, % | 58.9 [51.1-66.8] | 60.9 [53.1-68.7] | 60.9 [53.1-68.7] | 55.6 [47.7-63.6] | 66.1 [59.2-73.0] |
| Negative predictive value, % | 61.9 [54.3-69.4] | 63.8 [56.3-71.2] | 61.9 [54.3-69.4] | 68.8 [61.6-75.9] | 55.7 [47.2-64.2] |
| Positive likelihood ratio | 1.54 [1.22-1.95] | 1.67 [1.32-2.13] | 1.61 [1.27-2.05] | 1.66 [1.32-2.08] | 1.48 [1.20-1.83] |
| Negative likelihood ratio | 0.66 [0.53-0.83] | 0.61 [0.48-0.77] | 0.64 [0.51-0.80] | 0.60 [0.47-0.77] | 0.60 [0.46-0.78] |
| Diagnostic accuracy, % | 60.5 [60.3-60.6] | 62.4 [62.2-62.5] | 61.4 [61.3-61.6] | 62.4 [62.2-62.5] | 61.7 [61.6-61.9] |
| IVUS plaque burden | |||||
| Best cutoff value, % | 72.0 | 72.0 | 72.0 | 72.0 | 70.0 |
| Sensitivity, % | 66.7 [59.1-74.2] | 61.3 [53.5-69.1] | 61.4 [53.7-69.2] | 63.4 [55.3-71.6] | 70.6 [63.9-77.3] |
| Specificity, % | 61.5 [54.0-69.0] | 56.5 [48.9-64.2] | 57.0 [49.2-64.7] | 56.5 [49.2-63.8] | 56.7 [48.3-65.1] |
| Positive predictive value, % | 61.7 [54.2-69.2] | 56.8 [49.2-64.4] | 58.0 [50.4-65.6] | 52.5 [44.8-60.2] | 68.3 [61.6-75.0] |
| Negative predictive value, % | 66.4 [58.9-74.0] | 61.1 [53.2-68.9] | 60.4 [52.5-68.3] | 67.1 [59.6-74.7] | 59.4 [50.9-67.9] |
| Positive likelihood ratio | 1.73 [1.38-2.17] | 1.41 [1.14-1.75] | 1.43 [1.15-1.78] | 1.46 [1.18-1.80] | 1.63 [1.32-2.03] |
| Negative likelihood ratio | 0.54 [0.42-0.70] | 0.68 [0.54-0.87] | 0.68 [0.53-0.86] | 0.65 [0.50-0.84] | 0.52 [0.40-0.68] |
| Diagnostic accuracy, % | 64.0 [63.8-64.1] | 58.8 [58.7-59.0] | 59.2 [59.0-59.3] | 59.5 [59.3-59.6] | 64.6 [64.5-64.8] |
| IVUS percent area stenosis | |||||
| Best cutoff value, % | 60.0 | 60.0 | 60.0 | 60.0 | 57.0 |
| Sensitivity, % | 43.8 [35.5-52.1] | 30.7 [22.9-38.4] | 30.2 [22.6-37.8] | 31.4 [23.1-39.7] | 36.2 [28.8-43.6] |
| Specificity, % | 56.3 [48.4-64.2] | 78.1 [71.6-84.7] | 77.9 [71.2-84.5] | 77.8 [71.5-84.1] | 80.8 [73.9-87.7] |
| Positive predictive value, % | 47.6 [38.9-56.3] | 56.0 [44.8-67.2] | 56.0 [44.8-67.2] | 50.7 [39.4-62.0] | 71.1 [61.3-80.8] |
| Negative predictive value, % | 52.5 [44.8-60.2] | 55.4 [48.7-62.1] | 54.5 [47.5-61.1] | 61.0 [54.5-67.6] | 49.3 [42.4-56.1] |
| Positive likelihood ratio | 1.00 [0.77-1.30] | 1.40 [0.95-2.08] | 1.36 [0.92-2.02] | 1.42 [0.96-2.09] | 1.89 [1.25-2.85] |
| Negative likelihood ratio | 0.99 [0.81-1.22] | 0.89 [0.77-1.02] | 0.89 [0.78-1.03] | 0.88 [0.76-1.02] | 0.79 [0.68-0.91] |
| Diagnostic accuracy, % | 50.3 [50.2-50.5] | 55.6 [55.4-55.7] | 54.9 [54.7-55.0] | 58.3 [58.2-58.5] | 55.6 [55.4-55.7] |
| OCT minimum lumen area | |||||
| Best cutoff value, mm2 | 2.0 | 2.0 | 2.0 | 2.0 | 2.3 |
| Sensitivity, % | 63.0 [49.1-77.0] | 68.9 [55.4-82.4] | 65.3 [52.0-78.6] | 69.8 [56.0-83.5] | 70.9 [58.9-82.9] |
| Specificity, % | 57.8 [43.3-72.2] | 63.0 [49.1-77.0] | 61.9 [47.2-76.6] | 62.5 [48.8-76.2] | 61.1 [45.2-77.0] |
| Positive predictive value, % | 60.4 [46.6-74.3] | 64.6 [51.1-78.1] | 66.7 [53.3-80.0] | 62.5 [48.8-76.2] | 73.6 [61.7-85.5] |
| Negative predictive value, % | 60.5 [45.9-75.1] | 67.4 [53.4-81.4] | 60.5 [45.9-75.1] | 69.8 [56.0-83.5] | 57.9 [42.2-73.6] |
| Positive likelihood ratio | 1.49 [0.99-2.24] | 1.86 [1.22-2.85] | 1.71 [1.11-2.65] | 1.86 [1.23-2.82] | 1.82 [1.17-2.84] |
| Negative likelihood ratio | 0.64 [0.41-1.01] | 0.49 [0.30-0.80] | 0.56 [0.36-0.88] | 0.48 [0.29-0.80] | 0.48 [0.29-0.78] |
| Diagnostic accuracy, % | 60.4 [59.9-61.0] | 65.9 [65.5-66.4] | 63.7 [63.2-64.2] | 65.9 [65.5-66.4] | 67.0 [66.6-67.5] |
| OCT percent area stenosis | |||||
| Best cutoff value, % | 68.0 | 68.0 | 68.0 | 68.0 | 65.0 |
| Sensitivity, % | 56.5 [42.2-70.8] | 66.7 [52.9-80.4] | 59.2 [45.4-72.9] | 69.8 [56.0-83.5] | 67.3 [54.9-79.7] |
| Specificity, % | 55.6 [41.0-70.1] | 65.2 [51.5-79.0] | 59.5 [44.7-74.4] | 66.7 [53.3-80.0] | 55.6 [39.3-71.8] |
| Positive predictive value, % | 56.5 [42.2-70.8] | 65.2 [51.5-79.0] | 63.0 [49.1-77.0] | 65.2 [51.5-79.0] | 69.8 [57.5-82.2] |
| Negative predictive value, % | 55.6 [41.0-70.1] | 66.7 [52.9-80.4] | 55.6 [41.0-70.1] | 71.1 [57.9-84.4] | 52.6 [36.8-68.5] |
| Positive likelihood ratio | 1.27 [0.82-1.92] | 1.92 [1.23-2.99] | 1.46 [0.95-2.26] | 2.09 [1.34-3.27] | 1.51 [1.01-2.28] |
| Negative likelihood ratio | 0.78 [0.51-1.19] | 0.51 [0.32-0.81] | 0.69 [0.45-1.04] | 0.45 [0.28-0.75] | 0.59 [0.37-0.95] |
| Diagnostic accuracy, % | 56.0 [55.5-56.6] | 65.9 [65.5-66.4] | 59.3 [58.8-59.9] | 68.1 [67.7-68.6] | 62.6 [62.1-63.1] |
dPR, diastolic pressure ratio; FFR, fractional flow reserve; iFR, instantaneous wave-free ratio; IVUS, intravascular ultrasound; OCT, optical coherence tomography; Pd/Pa, distal to aortic coronary pressure; RFR, resting full-cycle ratio.
Values are expressed as median [interquartile range].
Regardless of IVUS or OCT-derived parameters, the overall diagnostic accuracy of the parameters was lower than 70% and the area under the curve were less than 0.75. In addition, the positive or negative likelihood ratios of anatomical parameters suggested that these parameters had limited diagnostic ability to predict functional significance of the target stenoses (table 3).
DiscussionThe current study evaluated the association between IVUS or OCT-derived quantitative parameters and invasive physiologic indices including new resting physiologic indices. The main findings were: a) all resting physiologic indices showed a significant correlation with quantitative parameters and showed a similar pattern of changes according to different ranges of IVUS or OCT-derived quantitative parameters; b) there was no difference in best cutoff values of IVUS or OCT-derived quantitative parameters for functionally significant stenosis defined by iFR, RFR, and dPR; c) FFR showed a stronger correlation with IVUS or OCT parameters than resting physiologic indices, and d) the best cutoff values of IVUS-MLA and OCT-MLA to define functionally significant stenosis were larger for FFR than those for resting physiologic indices. However, the overall diagnostic performance and discrimination indices of the severity of anatomical stenosis were modest in predicting functional significance defined by resting and hyperemic physiologic indices.
IVUS or OCT-derived parameters and resting physiologic indicesRecently, resting physiologic indices have been introduced into clinical practice and are under active investigation.20–22,31,32 Previous randomized controlled trials showed noninferiority of iFR-guided decisions for 1 year clinical outcome compared with FFR-guided decisions,31,32 and recent studies showed nearly identical diagnostic properties20–22 and similar prognostic implications22 between iFR and new resting indices, such as RFR and dPR. The current study evaluated the association between IVUS or OCT-derived anatomic plaque characteristics and the severity of functional stenosis defined by these resting and hyperemic physiologic indices. Across all the resting physiologic indices (iFR, RFR, or dPR), IVUS or OCT-derived parameters showed the same cutoff values and nearly identical diagnostic performance. In addition, resting physiologic indices showed similar patterns of changes according to IVUS or OCT-derived MLA, PB, and AS. In line with previous studies evaluating the diagnostic performance and prognostic implications of resting physiologic indices, the current results support that all the resting physiologic indices share the same property as a surrogate marker of stenosis severity assessed by IVUS and OCT. It is interesting that the best cutoff value of OCT-MLA was smaller than that of IVUS-MLA in predicting functional significance. This result is in line with previous studies, which demonstrated systematically lower measurement in OCT compared with IVUS.34
Resting and hyperemic physiologic indices for severity of anatomic stenosisIn the current study, both resting physiologic indices and FFR showed a significant correlation with the severity of anatomical stenosis assessed by IVUS or OCT. Although both resting physiologic indices and FFR significantly decreased with increased IVUS-PB/OCT-AS or decreased IVUS or OCT-MLA, FFR showed a higher correlation coefficient with IVUS or OCT-derived parameters and more sensitive changes to a different range of severity of anatomical stenosis. Furthermore, the cutoff values of those quantitative parameters for low FFR were observed in cases of less severe stenosis than resting physiologic indices. In previous studies by Lee et al.2 similar trends were also observed in which FFR decreased earlier than iFR, according to different % DS, hyperemic myocardial blood flow measured by 13N-ammonia PET, hyperemic stenosis resistance, and hyperemic trans-stenotic pressure gradient. These results explain why the iFR-guided revascularization strategy has resulted in less revascularization than the FFR-guided revascularization strategy in the DEFINE-FLAIR and iFR-SWEDEHEART trials.31,32 Considering that both iFR- and FFR-guided decisions showed a similar risk of 1-year clinical outcome, decisions based on resting physiologic indices might result in less revascularization with a similar risk of clinical events in patients undergoing revascularization.
Modest diagnostic performance of IVUS or OCT-derived parameters for defining functionally significant stenosisIn the current study, both IVUS and OCT-derived anatomic parameters showed a limited diagnostic performance and discriminant ability for both resting physiologic indices and FFR. These results are in line with previous studies evaluating the diagnostic performance of imaging parameters for low FFR.4–17 In previous studies evaluating the cutoff value of IVUS-MLA and their diagnostic performances to predict functionally significant stenosis defined by FFR, the cutoff values of MLA varied from 3 to 4mm2 across the studies,4–17 or according to the reference vessel size or lesion locations.8,9 Regardless of the cutoff values of MLA, diagnostic accuracies were around 70%. Similarly, Gonzalo et al.18 evaluated the association between OCT-MLA and FFR from 61 intermediate stenosis evaluated by OCT. In that study, OCT-MLA ≤ 1.95mm2 showed modest diagnostic accuracy (72%) and discrimination ability (area under the curve 0.74) and OCT-AS also showed limited diagnostic accuracy (57%) and discrimination ability (area under the curve 0.61). However, there has been no previous study that evaluated the cutoff values and diagnostic performance of IVUS or OCT to predict functional significance defined by resting physiologic indices.
As with the previous study, all the intravascular imaging-derived parameters in the current study showed <70% of diagnostic accuracy and <0.75 of area under the curve for both resting physiologic indices and FFR. These results imply that functional significance judged by invasive physiologic indices cannot be accurately predicted using anatomical information alone.2 The current study confirmed the limited predictability of anatomical information from invasive intravascular imaging for all the available physiologic indices from resting to hyperemic indices.
LimitationsThis study has several limitations. First, the current study evaluated the relationship between IVUS or OCT-derived parameters and invasive physiologic indices focusing on resting indices. Therefore, the current study is limited with respect to the impact of these modalities on clinical outcome. Second, as invasive physiologic indices are per-vessel indices and IVUS or OCT-derived parameters were for per-plaque assessment, the best cutoff value of IVUS or OCT-derived parameters cannot be applied to lesions with multiple stenoses. Third, although the current study mainly focused quantitative parameters for anatomic lesion severity, further study is warranted to explore the association between IVUS or OCT-defined qualitative plaque characteristics and invasive physiologic indices. Fourth, as the current study analyzed stenosis with the smallest MLA in cases of multiple stenoses in the target vessel, the results cannot be extrapolated to this clinical scenario. However, when the analysis was repeated after exclusion of cases with multiple stenoses (24/341, 6.6%), the overall results were not changed. Fifth, due to the retrospective nature of the current study, there may be a possibility of selection bias.
ConclusionsAll the resting physiologic indices showed an identical relationship with IVUS or OCT-defined quantitative plaque characteristics. FFR showed a stronger correlation with IVUS or OCT parameters than resting physiologic indices. The diagnostic accuracy and discrimination ability of anatomical parameters were modest in predicting functional significance defined by resting and hyperemic invasive physiologic indices.
CONFLICTS OF INTERESTJ.M. Lee received a research grant from St Jude Medical (Abbott Vascular) and Philips Volcano. J.-Y. Hahn received a Research Grant from St Jude Medical (Abbott Vascular) and Boston Scientific. B.-K. Koo received an Institutional Research Grant from St Jude Medical (Abbott Vascular) and Philips Volcano. All other authors declare that there is no conflict of interest relevant to the submitted work.
- –
Since the development of iFR, several resting pressure-derived physiologic indices, such as RFR and dPR, have been introduced in daily practice. However, there are limited data regarding the association between IVUS or OCT-derived quantitative plaque characteristics and physiologic stenosis severity assessed by resting indices.
- –
All the resting physiologic indices showed an identical relationship with IVUS or OCT-defined quantitative plaque characteristics. The diagnostic accuracy and discrimination ability of anatomical parameters were modest in predicting functional significance defined by resting and hyperemic invasive physiologic indices. These results imply that any type of resting index can be used interchangeably in daily practice to guide treatment decisions and also provide evidence that functional significance judged by invasive physiologic indices cannot be accurately predicted using anatomical information alone.
Supplementary data associated with this article can be found in the online version available, at https://doi.org/10.1016/j.rec.2019.11.001.