Symptomatic mitral regurgitation has an unfavorable prognosis unless treated by surgery. However, the European registry of valvular heart disease reports that 49% of patients with this condition do not undergo surgery. Percutaneous treatment of mitral regurgitation with MitraClip® has been proved a safe, efficient adjunct to medical treatment in patients with this profile. The objective of the present study is to describe initial experience of MitraClip® therapy in Spain.
MethodsRetrospective observational study including all patients treated between November 2011 and July 2013 at the 4 Spanish hospitals recording the highest numbers of implantations.
ResultsA total of 62 patients (77.4% men) were treated, mainly for restrictive functional mitral regurgitation (85.4%) of grade III (37%) or grade IV (63%), mean (standard deviation) ejection fraction 36% (14%), and New York Heart Association functional class III (37%) or IV (63%). Device implantation was successful in 98% of the patients. At 1 year, 81.2% had mitral regurgitation ≤ 2 and 90.9% were in New York Heart Association functional class ≤ II. One periprocedural death occurred (sepsis at 20 days post-implantation) and another 3 patients died during follow-up (mean, 9.1 months). Two patients needed a second implantation due to partial dehiscence of the first device and 2 others underwent heart transplantation.
ConclusionsIn Spain, MitraClip® therapy has principally been aimed at patients with functional mitral regurgitation, significant systolic ventricular dysfunction, and high surgical risk. It is considered a safe alternative treatment, which can reduce mitral regurgitation and improve functional capacity.
Keywords
Mitral regurgitation is the second most frequent valvular heart disease in the developed world.1 When mitral regurgitation is severe and accompanied by clinically demonstrated left-sided heart failure, the prognosis without surgical treatment is unfavorable. High mortality at 5 years has even been reported in asymptomatic patients with severe degenerative mitral regurgitation.2,3 Surgical treatment is considered a class I indication for asymptomatic patients with severe mitral regurgitation or those presenting with progressive dilatation or left ventricular dysfunction, and valve repair is preferred over replacement.4 However, 49% of patients included in the Euro Heart Survey on Valvular Heart Disease with class I indication for surgery are not operated on because they are considered as presenting high surgical risk,5 mainly due to advanced age, systolic ventricular dysfunction, and comorbidities.
In recent years, new treatments of mitral regurgitation have been developed. These use a percutaneous approach as an alternative to surgery for patients at high surgical risk.6 Among them, the most thoroughly developed approach imitates the Alfieri technique by positioning a cobalt chromium device (MitraClip®, Abbot Vascular) in the center of the valve so that it grasps both leaflets and holds them together. Thus, a diastolic–filling double orifice is created and the degree of regurgitation is reduced. The EVEREST I study7 proved this treatment was both feasible and safe. Later, the randomized EVEREST II study8 demonstrated its efficacy (although surgery continued to provide better results), with 72% of patients having residual mitral regurgitation ≤ 2 at 1-year follow-up. In 2008, the MitraClip® system received CE mark approval and since then more than 10 000 devices have been implanted, mainly in Europe. The present study is the first multicenter registry of our experience with the MitraClip® system in Spain.
Our objective is to describe the profile of the first MitraClip® recipients in Spain and to determine the early efficacy of the treatment and complications associated with implantation. We also describe the clinical course of patients at 1-year follow-up.
METHODSRetrospective observational study, using data provided voluntarily by the 4 Spanish hospitals with the highest numbers of implantations. The participating centers are Hospital de la Santa Creu i Sant Pau (Barcelona), Hospital Reina Sofía (Córdoba), Hospital Clínico de Valladolid (Valladolid), and Hospital Virgen de la Victoria (Málaga).
Patient EnrollmentWe included all patients treated in the participating hospitals between November 2011 and July 2013. Device implantation was indicated in patients with symptomatic grade III–IV mitral regurgitation despite optimal medical treatment, who were classified as being at high surgical risk by the medical-surgical team at each of the participating centers, and who adequately fulfilled the anatomic criteria making implantation feasible, using the EVEREST study guidelines.7,8 Patients underwent prior transesophageal echocardiography and the images were reviewed by a committee of external experts from Abbott Vascular. All patients received oral and written information about the risks and benefits of the procedure and gave written informed consent. Each hospital collected its own data and transferred this to a common database.
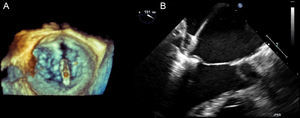
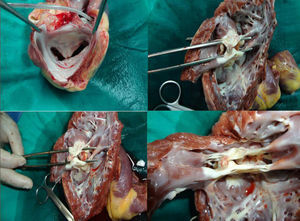
Description of the ProcedureAll procedures were performed under general anesthesia and guided by 3-dimensional transesophageal echocardiography in consultation with a team of specialists from MitraClip®. The teams involved in these procedures consisted of, at least, 2 interventional cardiologists, 1 echocardiographer, 1 anesthetist, and nursing staff with experience in the technique. Procedures were performed via femoral vein puncture and access to the left atrium was by transseptal puncture. Transseptal puncture was guided by transesophageal echocardiography and made precisely in the upper posterior portion of the fossa ovalis, ensuring that the distance between the point of puncture and the valve coaptation line measured between 4cm and 5cm (in a 4–chamber plane). On a high-support guidewire, a 24 Fr catheter guidewire was advanced to the left atrium and the MitraClip® device passed through this. Until it reached the left ventricle, all movement of the clip in the left atrium was guided by 3-dimensional echocardiography. The clip arms were opened to 120° and the device advanced into the left ventricle; the clip was then aligned perpendicular to the valve coaptation plane (Figure 1A). The open clip was withdrawn towards the left atrium, grasping the mitral valve leaflets, which were held together by the closed clip (Figure 1B). After the leaflets had been gathered together, the results (degree mitral regurgitation, mitral gradient, mitral valve area, and length of leaflets held by the clip) were analyzed in the echocardiogram. The decision was then taken either to definitively deploy the clip or reposition it to improve results. To optimize results, more than one clip was implanted if there was no residual mitral stenosis.
Definition of VariablesEchocardiographicIn the catheterization laboratory, procedure success was defined as the reduction of mitral regurgitation to grade ≤ II after deploying the clip. This was determined by transesophageal echocardiography. Doppler quantification was done according to American Society of Echocardiography recommendations.9 Transthoracic echocardiography was used to quantify mitral regurgitation prior to implantation and during follow-up, in line with the aforementioned recommendations. During follow-up, grade III–IV mitral regurgitation was classified as significant.
ClinicalThe patients’ functional capacity was assessed subjectively using the New York Heart Association (NYHA) scale. Admission for heart failure was defined as hospitalization for treatment or attending the Emergency Room and needing to be admitted overnight. The logistic EuroSCORE was calculated. Hospitalization, heart transplantation, reintervention, and any-cause death were considered as events during follow-up.
Statistical AnalysisCategorical variables are presented as percentages. Continuous variables are presented as mean (standard deviation). To establish associations between categorical variables, relative risk has been calculated using chi-square and the 95% confidence interval (95%CI) of relative risk. To establish associations between continuous variables, after checking that they adjust to a normal distribution, the difference between means has been measured; the Student t test was used for paired samples and the 95% CI for the difference of means. We constructed Kaplan-Meier curves to study event-free survival. We accepted a maximum alpha error of 0.05. Data analysis was done with R software.
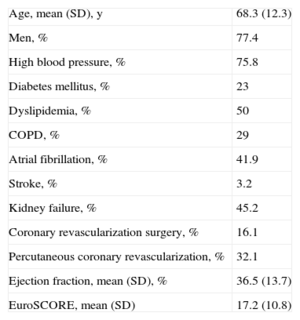
RESULTS1.1Characteristics of the PopulationIn the participating hospitals, a total of 62 patients received MitraClip® devices between November 2011 and July 2013. Demographic data are shown in Table 1.
Baseline Clinical Characteristics of the 62 Patients Treated
| Age, mean (SD), y | 68.3 (12.3) |
| Men, % | 77.4 |
| High blood pressure, % | 75.8 |
| Diabetes mellitus, % | 23 |
| Dyslipidemia, % | 50 |
| COPD, % | 29 |
| Atrial fibrillation, % | 41.9 |
| Stroke, % | 3.2 |
| Kidney failure, % | 45.2 |
| Coronary revascularization surgery, % | 16.1 |
| Percutaneous coronary revascularization, % | 32.1 |
| Ejection fraction, mean (SD), % | 36.5 (13.7) |
| EuroSCORE, mean (SD) | 17.2 (10.8) |
COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Unless otherwise indicated, figures are expressed as mean (standard deviation).
The most important baseline characteristic of the population is the widely varying ages of patients: 19.4% were<60 years old at implantation. Mean ejection fraction was 36.5% (13.7%).
The etiology of mitral regurgitation was functional in 85.4% of recipients and degenerative in 11.3%. In 3.3%, the etiology was mixed.
The mechanism of regurgitation was Carpentier type III (restrictive) in 84.5% of patients. In 63.8%, restriction of both mitral leaflets was symmetrical, and in 20.7% it was asymetrical, with the posterior leaflet being the more markedly restricted. Some 15.5% of recipients had valve prolapse (Carpentier type II mechanism).
Data on the ProcedureEssentially, in these patients the principle regurgitation jet was situated over the A2/P2 segments of the mitral valve (95.2% of patients). In 1 patient, the principle regurgitation jet was over the A1/P1 segments and in another it was over the A3/P3 segments. In 14.5%, we identified>1 significant jet.
Mitral regurgitation was considered to be grade IV in 63% of patients and grade III in 37%. In the echocardiography study prior to treatment, mean systolic pulmonary pressure was 57.4 (19.1) mmHg.
A total of 61% of patients received a single clip and 35% received 2 clips. One patient had 3 clips implanted and 1 patient received 4. In the catheterization laboratory, transesophageal echocardiography studies found that 98% of patients had grade ≤ 2 mitral regurgitation on completing the procedure.
Two patients had potentially severe complications during the procedure. One required pericardiocentesis due to cardiac taponade, without needing surgery and with an ultimately successful clinical course. In another patient, a retroaortic hematoma appeared following transseptal puncture, and we discovered a fistula between the right atrium and the aorta, with a maximum gradient of 56mmHg. This received conservative management and final clinical course was good.
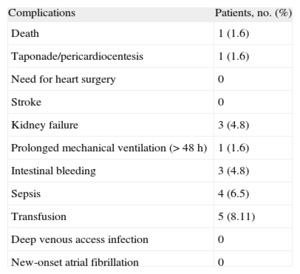
Follow-up DataComplications occurred in 6 patients during hospitalization and in the first month of post-implantation follow-up. These included 1 death at 20 days due to septic shock, 2 cases of nosocomial urinary tract infections (1 complicated by septic shock and digestive bleeding), 1 case of digestive bleeding, 1 pneumonia, and 1 case of post-procedural arrhythmic storm. Table 2 presents a breakdown of complications or adverse events during hospitalization or in the first month of follow-up. Note that no cases of stroke associated with device implantation have been reported and none of the patients needed urgent heart surgery during the post-implantation hospital stay.
Breakdown of Complications Associated With Device Implantation and Recorded During Hospitalization and the First Month of Follow-up (n=62)
| Complications | Patients, no. (%) |
| Death | 1 (1.6) |
| Taponade/pericardiocentesis | 1 (1.6) |
| Need for heart surgery | 0 |
| Stroke | 0 |
| Kidney failure | 3 (4.8) |
| Prolonged mechanical ventilation (> 48 h) | 1 (1.6) |
| Intestinal bleeding | 3 (4.8) |
| Sepsis | 4 (6.5) |
| Transfusion | 5 (8.11) |
| Deep venous access infection | 0 |
| New-onset atrial fibrillation | 0 |
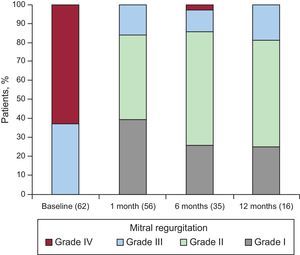
Mean follow-up was 9.1 months. Figure 2 shows the grade of mitral regurgitation at baseline and at 1, 6, and 12 months follow-up. The rates of grade ≤ II mitral regurgitation at 1, 6, and 12 months were 83.9%, 85.7%, and 81.2% respectively.
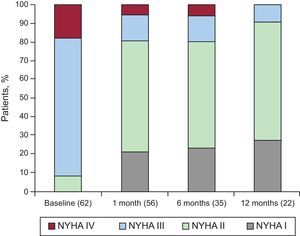
Figure 3 shows NYHA functional class at baseline and at 1, 6, and 12 months. The percentages of patients in NYHA ≤ 2 at 1, 6, and 12 months of follow-up were 80.75%, 80%, and 90.9%, respectively.
Ejection fraction at 6 months was 38.1%, which was not significantly different from the previous value: difference of means, 1.97 (95%CI, –5.6 to 1.75; P=.29). In 8 patients, mean pulmonary pressure was measured at 12-month follow-up (mean, 43mmHg). Previously, mean pulmonary pressure in these patients had been 60.6%, representing a reduction of 17.6mmHg (95%CI, 7.9-27.3; P=.004).
During follow-up, 20% of patients needed readmission due to heart failure, which occurred, on average, in the fifth month. Half of the patients who were readmitted had obtained a good reduction in mitral regurgitation (residual mitral regurgitation ≤ 2 at 1 month). Moreover, the relation between this good result and need for rehospitalization during the follow-up was statistically significant (relative risk=0.19; 95%CI, 0.07-0.46; P<.01). Two patients needed to have a second MitraClip® device implanted because of the partial dehiscence of the first device.
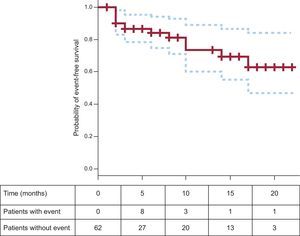
Figure 4 shows the Kaplan-Meier curve for event-free survival (death, transplantation, admission, reintervention). Two patients underwent heart transplantation (Figure 5) due to severe heart failure with frequent decompensation despite optimal medical treatment. Four deaths have been recorded—1 of which was due to a cardiovascular cause—at a mean 7.9 months post-implantation.
The present study reports initial experience of MitraClip® therapy in the 4 hospitals with the highest numbers of implantations in Spain.10 Knowledge of percutaneous treatment of mitral regurgitation with the MitraClip® device comes from the EVEREST studies and the recently-published European registries. The EVEREST II study included 258 patients (randomized 2:1; MitraClip® vs surgery) with severe mitral regurgitation, which was degenerative in 74% of cases, mainly with mean ejection fraction preserved.8 Note that at 4 years follow-up,11 differences in mortality were nonexistent (17.4% among MitraClip® recipients vs 17.8% in the surgery group; P=.91), although need for reintervention was significantly greater among the MitraClip® patients (24.8% vs 5.5%; P<.001).8 Reinterventions in MitraClip® recipients mainly took place at ≤ 1 year and, subsequently, only 4.4% of patients needed reintervention. This indicates that when successful—in approximately 3 out of 4 patients—MitraClip® therapy has acceptable durability. Among MitraClip® recipients subsequently needing surgery, 54% underwent valve repair and the rest needed valve replacement. In our series too, nearly 20% of patients had severe or moderate-severe mitral regurgitation at 1-year follow-up, although reintervention was not performed because they had not initially been indicated for surgery. Only 2 patients underwent new device implantation procedures due to dehiscence of the first device.
In real life, the patient profile—to judge by that reported in the present and other observational studies—is radically different from that of the EVEREST study. In Spain, and this can be extrapolated to Europe, MitraClip® recipients have mainly been patients with functional mitral regurgitation, rather than degenerative mitral regurgitation, and significant systolic ventricular dysfunction, with a high EuroSCORE. In fact, the patients receiving a MitraClip® implant have been those for whom surgery was considered inviable due to their high risk level and because they remained symptomatic despite optimal medical treatment. If the EVEREST studies compared MitraClip® with surgery, in real life, far from competing with surgery, MitraClip® therapy has come to fill the gap left when surgery cannot produce good results. Randomized studies are currently under way. For example, RESHAPE12 is comparing percutaneous treatment with MitraClip® vs medical treatment and could provide definitive results as to what MitraClip® can offer these patients.13–15 Until then, we have access to registries which appear to present positive results.16 The ACCESS EU study of 567 patients reports highly effective treatment: 78% have mitral regurgitation ≤ II at 1 year and 71.4% are in NYHA I-II—moreover, incidence of device-related complications is low (30-day mortality, 3.4%). In Italy, the single-center GRASP registry,17 with 117 patients (76% with functional mitral regurgitation and mean ejection fraction 38%), reports residual mitral regurgitation ≤ 2 at 1 year in 93% of patients with functional etiology and in 75% of patients with degenerative etiology, and 3.4% of associated major adverse events at 30 days (notably 1 death and 1 case of stroke). Approximately 3 of 4 patients report improvements in functional class and in distance walked on the 6-min walk test.18–20 The results of our series are very similar to those presented in these registries, both in recipient profile, following the European Society of Cardiology clinical guidelines’ recommendations on valvular heart disease and heart failure,4 and in results and implantation-related complications: 81.2% with mitral regurgitation ≤ II at 1 year follow-up and only 1 early death. Moreover, the series reflects the learning curve this procedure entails—probably the most technically complex procedure in structural heart disease. Note the discrepancy between residual mitral regurgitation measured in the catheterization laboratory immediately after implantation (only in 2% of cases>II) and that measured at 1 month (approximately 1 out of 6 patients presents significant mitral regurgitation, of grade III-IV). This is probably related to the physiologic conditions in which regurgitation is measured in the former context (orotracheal intubation and tendency for arterial hypotension due to the vasodillatory effects of the anesthesia). More striking than the results of echocardiography is the improvement in functional status, as 4 of 5 patients are in NYHA II even though mean ejection fraction was 36%. Notwithstanding, the percentage of decompensations requiring admission rises to 20% after 1 year of follow-up. As in the early days of resynchronization therapy, it appears we can identify some patients who progress slowly despite the reduction in mitral regurgitation and this, presumably, is related to depressed left ventricular function. At the time of writing, we lack validated tools that help predict poor progress in MitraClip® recipients with dilated cardiomyopathy and severe left ventricular dysfunction. Hence, studies enrolling large numbers of patients will be needed to enable us to identify them.16
LimitationsData collection was retrospective and follow-up sometimes differed between centers.
Functional capacity was measured using the NYHA classification but quantitative tests capable of objective measurement of functional capacity were not uniformly done in all patients.
CONCLUSIONSTreatment with MitraClip® therapy in Spain has concentrated on patients with functional mitral regurgitation and high surgical risk and is profiled as a safe optional treatment facilitating the reduction of mitral regurgitation and improving functional capacity.
CONFLICTS OF INTERESTNone declared.