Neoatherosclerosis is one of the causes of in-stent restenosis (ISR). Our objective was to evaluate the influence of neoatherosclerosis on prognosis and treatment response in patients with ISR.
MethodsThis is a pooled analysis of the optical coherence tomography (OCT)-substudies of 2 multicenter, randomized clinical trials, RIBS IV and V, comparing treatment with paclitaxel-coated balloon vs everolimus-eluting stent in patients with ISR. OCT evaluation was performed at baseline and at 6 to 9 months. Neoatherosclerosis was defined in baseline OCT as neointima with calcified or lipid content. We evaluated the angiographic and OCT results at 6 to 9 months and the occurrence of major adverse cardiovascular events at 3 years of follow-up in patients with and without neoatherosclerosis treated with paclitaxel-coated balloon or everolimus-eluting stents.
ResultsSixty-four patients underwent OCT at the time of the index procedure. Neoatherosclerosis was documented in 23 (36%) lesions. Angiographic follow-up at 6 to 9 months showed no differences in restenosis [5 (24%) vs 6 (15%) P=.49], minimum lumen diameter (1.79±0.7 vs 1.94±0.6mm; P=.41) or late loss (0.33±0.7 vs 0.15±0.5; P=.34) in patients with and without neoatherosclerosis, respectively. Follow-up OCT confirmed the absence of differences in quantitative parameters and the characteristics of tissue coverage between the 2 groups. At 3 years of follow-up, the major adverse cardiovascular events rate was 3 (13%) vs 5 (12%) in the neoatherosclerosis and nonneoatherosclerosis groups (HR, 0.94; 95%CI, 0.22-3.93; P=.93).
ConclusionsIn this limited study population, OCT-defined neoatherosclerosis did not seem to influence acute and long-term outcomes in patients randomized to paclitaxel-coated balloon or everolimus-eluting stents for ISR.
Keywords
Progress in coronary stent technology has dramatically reduced the rates of restenosis. However, this type of stent failure remains a clinical problem even with new generation drug-eluting stents. Further developments in its prevention may require a deeper understanding of the underlying pathology.
In vivo interrogation of in-stent restenosis (ISR) with optical coherence tomography (OCT), a high-resolution intracoronary imaging tool, has revealed that the structure of ISR is frequently heterogenous.1 In addition to showing different patterns of ISR,1 OCT has demonstrated that, not infrequently, luminal obstruction in ISR is caused by neoatherosclerosis2,3 and not, as previously thought, by homogenous fibrous hyperplasia. Little is known, however, about whether neoatherosclerosis influences long-term outcomes in patients with ISR treated with percutaneous coronary intervention (PCI), or whether paclitaxel-coated balloon (PCB) or everolimus-eluting stents (EES) are equally effective in treating ISR with neoatherosclerosis. Therefore, the objectives of this study were a) to evaluate the influence of OCT-defined neoatherosclerosis on acute and long-term outcomes in patients with ISR treated with either PCB or EES; and b) to assess the response of neoatherosclerosis to treatment with PCB or EES.
METHODSStudy populationWe performed a pooled analysis of patients included in the predefined OCT-substudies of the RIBS IV and V, prospective multicenter, controlled, randomized clinical trials, which compared treatment with PCB vs EES in patients with either drug-eluting stent (DES)-ISR (RIBS IV) or bare metal stent-ISR (RIBS-V).4,5 ISR was defined as the presence of a >50% lumen diameter stenosis on visual assessment at the site of a previously implanted stent or involving its 5-mm edges. Angina or evidence of ischemia was a prerequisite for study inclusion. We excluded ISR in small vessels (reference vessel diameter <2.0mm), diffuse ISR patterns (> 30mm in length) and ISR presenting as chronic total occlusions. Other exclusion criteria were life expectancy <1 year, inability to perform angiographic follow-up, situations precluding the use of OCT (severe angiographic tortuosity or renal insufficiency), inability to maintain 1-year dual antiplatelet therapy, and evidence of stent thrombosis
Patients were randomized 1:1 to EES or PCB using a computer-generated randomization code. Randomization was stratified according to ISR length (< or> 10mm) and lesion location (intrastent vs edge-ISR). Randomization, data monitoring, management and analysis was performed at the coordinating center.
The study was approved by the institutional Ethics Committees of all sites and followed the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Treatment protocolBefore intervention, patients were treated with dual antiplatelet therapy and unfractionated heparin was used during the procedure. Optimal lesion preparation was mandated by the protocol before using EES or PCB. Predilatation had to be performed avoiding damaging the adjacent segments and ensuring an adequate expansion of the restenotic stent. After lesion preparation, the patients were treated according to randomization with PCB or EES. Treatment with PCB was performed with a paclitaxel-eluting balloon SeQuent Please (B. Braun Surgical, Germany) using a 1.1/1 balloon-to-artery ratio and 60-second inflation at nominal pressure. EES (Xience Prime, Abbott Vascular, United States) were implanted at high pressure with a 1.1/1 final balloon-to-artery ratio. Postdilatation was recommended after EES implantation but was left to the operator's discretion.
Follow-upAngiographic follow-up was scheduled at 6 to 9 months. Clinical follow-up was obtained at 6 to 9 months, 12 months and then on a yearly basis.4,5 Data acquisition was monitored by the coordinating center. Adjudication of clinical events (death, myocardial infarction, target vessel/lesion revascularization) was performed by an independent Clinical Events Committee blinded to the treatment arm. Clinical events were defined as follows: a) cardiac death: all deaths were considered cardiac unless a noncardiac cause could be documented. b) myocardial infarction: presence of 2 of the following: prolonged (> 30minutes) chest pain, rise in creatine-kinase levels> 2-fold the local upper normal limits (with abnormal myocardial band fraction), or development of persisting ischemic electrocardiogram changes (with or without new pathological Q waves). c) Target vessel revascularization: new revascularization in the target vessel. d) Target lesion revascularization: new revascularization in the target lesion. All interventions performed at follow-up had to be clinically indicated (symptoms or ischemia demonstrated in invasive or noninvasive tests). The Academic Research Consortium criteria were used to define stent thrombosis.
OCT acquisition and analysisThe OCT substudy was performed in a group of the centers participating in the RIBS IV and V trial and included OCT evaluation at baseline and at 6 to 9 months during the angiographic follow-up. OCT was acquired using the C7XR system and the Dragonfly catheter (Light Lab, St Jude Medical, United States) with a nonocclusive technique. Offline analysis was performed with QIVUS (Medis, Netherlands). The analyzed segment included the stent region and the stent margins defined as the vessel segment 5mm proximal and distal to the stent. Measurements were performed at 1-mm longitudinal steps throughout the pullback. Qualitative parameters were analyzed by agreement between 2 observers blinded for the clinical and procedural characteristics.
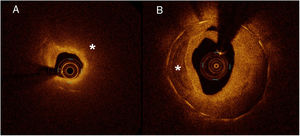
Neoatherosclerosis was defined in the baseline OCT as the presence of restenotic tissue with calcification (well-delineated, signal-poor region with sharp borders) or lipid content (signal-poor region with diffuse borders)6 (figure 1). We also evaluated other parameters such as the restenotic tissue structure (homogeneous, heterogenous, layered), restenotic tissue backscatter, peristrut low-intensity area (PSLIA), presence of microvessels (well-delineated low backscattering structures <200μm in diameter showing a trajectory within the vessel), presence of macrophages (identified as signal rich, distinct or confluent punctuate regions with a linear dorsal shadowing), lumen shape and presence of intraluminal material. Baseline OCT analysis included a quantitative analysis of the lumen area, stent area, stent expansion (minimum stent area/reference stent area) and stent symmetry (minimum/maximum stent area). The restenotic tissue area was defined as stent area minus lumen area and the restenotic tissue burden was calculated as mean restenotic tissue area/mean stent area×100. The cross section containing the minimum luminal area was used to determine the maximum and minimum restenotic tissue thickness and the restenotic tissue symmetry ratio (maximum restenotic tissue thickness − minimum restenotic tissue thickness/maximum restenotic tissue thickness).
Neoatherosclerosis. A: restenotic tissue with lipid content (signal-poor region with diffuse borders indicated by *). The example shows a case with intense light attenuation that precludes visualization of struts (they are visible only from 6 to 11). B: restenotic tissue with calcification (well-delineated, signal-poor region with sharp borders indicated by *).
At follow-up, OCT measurements of lumen area, stent area and stent expansion, tissue coverage area and tissue coverage burden were performed. In the cross section with the highest tissue coverage burden, the tissue coverage structure and backscatter, presence of microvessels, lumen shape and presence of intraluminal material were evaluated with the same definitions as those used for the baseline evaluation of restenotic tissue. In patients treated with PCB, we evaluated the tissue coverage above the struts of the previously implanted stent. Finally, we also assessed the presence of uncovered struts (no visible layer of tissue overlying its bright signal-intense structure), evaginations (outward bulges in the luminal contour between struts extending ≥ 3mm along the vessel length, with a depth ≥ 10% of the stent diameter) and PSLIAs (homogenous low-intensity area around a stent strut without significant signal attenuation behind the area).7,8
Statistical analysisCategorical data were compared with the chi-square test or the Fisher exact test as required. Data distribution normality was evaluated with the Kolmogorov-Smirnov test. Continuous data expressed as mean±standard deviation or median (interquartile range were compared using the Student t-test or the Mann-Whitney test. Main effect estimates are presented with their 95% confidence intervals. Event estimates are expressed as Kaplan-Meier estimates of the cumulative incidence at 1 year and were compared with the Log-rank and Breslow exact tests. Hazard ratios (95% confidence interval) were generated with the use of Cox proportional-hazard models and compared with the Wald test. The SPSS statistical package (version 15.00) was used. All reported P values were 2-sided and a P value <.05 was considered as statistically significant.
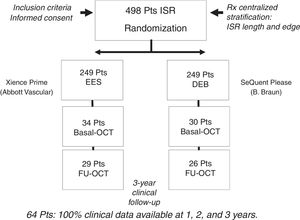
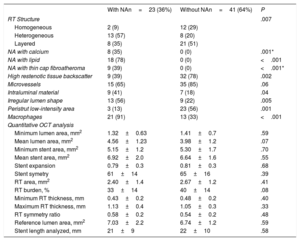
RESULTSBaseline clinical, angiographic and OCT characteristics of ISR with neoatherosclerosisFigure 2 shows how the OCT substudy population analyzed in this research (n=64) was extracted from the pooled RIBS IV and V trials (n=498). There were no differences in baseline or angiographic characteristics between patients with or without OCT evaluation (table 1 and table 2 of the supplementary data). Neoatherosclerosis was present in 23 (36%) lesions, being more frequent in DES [16 (70%)] than in bare-metal stents [7 (30%)] (P=.15). The presence of neoatherosclerosis was not linked to baseline characteristics including age, sex, or risk factors. Patients with neoatherosclerosis more frequently presented with unstable angina [15 (65%) vs 11 (27%)] for patients with and without neoatherosclerosis, respectively (P=.003) and had a longer time from stent implantation: 1117 (270-2772) vs 270 (200-1075) days for patients with and without neoatherosclerosis, respectively, P=.01 (table 1).
Baseline clinical and angiographic characteristics
| With NAn=23 (36%) | Without NAn=41 (64%) | P | |
|---|---|---|---|
| Age, y | 64±11 | 66±12 | .42 |
| Female sex | 3 (13) | 8 (20) | .73a |
| Risk Factors | |||
| Diabetes mellitus | 9 (39) | 13 (32) | .55 |
| Hyperlipidemia | 16 (70) | 25 (61) | .49 |
| Hypertension | 18 (78) | 26 (63) | .22 |
| Ever smoker | 14 (61) | 29 (71) | .42 |
| Clinical Features | |||
| Clinical presentation | .003 | ||
| Unstable angina | 15 (65) | 11 (27) | |
| Stable angina/silent ischemia | 8 (35) | 30 (73) | |
| Previous myocardial infarction | 13 (57) | 28 (68) | .35 |
| Previous bypass surgery | 2 (9) | 1 (2) | .29a |
| Time to restenosis, d | 1117 [270-2772] | 270 [200-1075] | .01b |
| (Median/mean) | 1585±1433 | 895±1211 | |
| Ejection fraction, % | 62±13 | 62±14 | .90 |
| Target artery | .50 | ||
| Left anterior descending | 11 (48) | 17 (42) | |
| Left circumflex | 7 (30) | 9 (22) | |
| Right coronary | 5 (22) | 15 (37) | |
| Previous stent type | .15 | ||
| BMS | 7 (30) | 20 (49) | |
| DES | 16 (70) | 21 (51) | |
| Length previous stent, mm | 22±8 | 19±7 | .12 |
| Maximal pressure, atm | 20±5 | 21±4 | .64 |
| Angiographic characteristics | |||
| Mehran I, II, III-IV | 18 (78),4 17), 1 (4) | 18 (44),18 (44), 5 (12) | .03 |
| B2-C lesion | 10 (44) | 24 (59) | .25 |
| Edge-ISR | 7 (30) | 7 (17) | .22 |
| Reference vessel diameter, mm | 2.62±0.5 | 2.49±0.5 | .31 |
| Minimum lumen diameter, mm | 0.82±0.3 | 0.74±0.3 | .37 |
| Stenosis (% of lumen diameter) | 67±13 | 70±13 | .40 |
| Lesion length, mm | 11.9±7 | 13.6±6 | .35 |
BMS, bare metal stent; DES, drug-eluting stent; ISR, in-stent restenosis; NA, neoatherosclerosis.
The results are expressed as No. (%), mean±standard deviation, or median [interquartile range].
ISR with neoatherosclerosis more frequently presented with a Mehran I angiographic pattern [18 (78%) vs 18 (44%) P=.03]. There was a nonsignificant tendency toward more edge restenosis in the neoatherosclerosis group [7 (30%) vs 7 (17%) P=.22]. There were no differences in other baseline angiographic parameters (minimum lumen diameter, % diameter stenosis, lesion length, reference vessel diameter) (table 1).
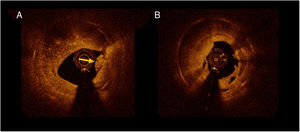
Significant differences in the structure of ISR, as judged with OCT imaging, were noted between ISR lesions with and without neoatherosclerosis. A heterogeneous pattern was more frequently found in lesions with neoatherosclerosis [13 (57%) vs 8 (20%)], in ISR without neoatherosclerosis; P=.007) as well as a low backscatter [14 (61%) vs 9 (22%) P=.002]. Neoatherosclerosis cases more frequently had an irregular lumen [13 (56%) vs 9 (22%) P=.005], visible intraluminal material [9 (41%) vs 7 (18%) P=.04] and macrophages [21 (91%) vs 13 (33%) P <.001] (figure 3). No significant differences in the prevalence of neointimal microvessels was noted in lesions with and without neoatherosclerosis. PSLIA were less often observed in neoatherosclerosis [3 (13%) vs 23 (56%) P=.001]. No differences were observed in OCT measurements of lumen and stent areas, stent expansion, and symmetry. There was a tendency toward a lower restenotic tissue burden in the neoatherosclerosis group (33%±14% vs 40%±14% P=.08) (table 2).
Baseline optical coherence tomography
| With NAn=23 (36%) | Without NAn=41 (64%) | P | |
|---|---|---|---|
| RT Structure | .007 | ||
| Homogeneous | 2 (9) | 12 (29) | |
| Heterogeneous | 13 (57) | 8 (20) | |
| Layered | 8 (35) | 21 (51) | |
| NA with calcium | 8 (35) | 0 (0) | .001* |
| NA with lipid | 18 (78) | 0 (0) | <.001 |
| NA with thin cap fibroatheroma | 9 (39) | 0 (0) | <.001* |
| High restenotic tissue backscatter | 9 (39) | 32 (78) | .002 |
| Microvessels | 15 (65) | 35 (85) | .06 |
| Intraluminal material | 9 (41) | 7 (18) | .04 |
| Irregular lumen shape | 13 (56) | 9 (22) | .005 |
| Peristrut low-intensity area | 3 (13) | 23 (56) | .001 |
| Macrophages | 21 (91) | 13 (33) | <.001 |
| Quantitative OCT analysis | |||
| Minimum lumen area, mm2 | 1.32±0.63 | 1.41±0.7 | .59 |
| Mean lumen area, mm2 | 4.56±1.23 | 3.98±1.2 | .07 |
| Minimum stent area, mm2 | 5.15±1.2 | 5.30±1.7 | .70 |
| Mean stent area, mm2 | 6.92±2.0 | 6.64±1.6 | .55 |
| Stent expansion | 0.79±0.3 | 0.81±0.3 | .68 |
| Stent symetry | 61±14 | 65±16 | .39 |
| RT area, mm2 | 2.40±1.4 | 2.67±1.2 | .41 |
| RT burden, % | 33±14 | 40±14 | .08 |
| Minimum RT thickness, mm | 0.43±0.2 | 0.48±0.2 | .40 |
| Maximum RT thickness, mm | 1.13±0.4 | 1.05±0.3 | .33 |
| RT symmetry ratio | 0.58±0.2 | 0.54±0.2 | .48 |
| Reference lumen area, mm2 | 7.03±2.2 | 6.74±1.2 | .59 |
| Stent length analyzed, mm | 21±9 | 22±10 | .58 |
NA, neoatherosclerosis; OCT, optical coherence tomography; RT, restenotic tissue.
The data are expressed as No. (%) or mean±standard deviation.
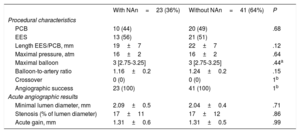
There were no differences between patients with and without neoatherosclerosis regarding treatment strategy. The same proportion in each group were treated with PCB or EES and no differences were observed in any other procedural aspects (balloon diameter, pressure, length of the new EES, or PCB). The angiographic acute gain and minimum lumen diameter was also similar between the 2 groups (table 3).
Procedural characteristics and acute angiographic results
| With NAn=23 (36%) | Without NAn=41 (64%) | P | |
|---|---|---|---|
| Procedural characteristics | |||
| PCB | 10 (44) | 20 (49) | .68 |
| EES | 13 (56) | 21 (51) | |
| Length EES/PCB, mm | 19±7 | 22±7 | .12 |
| Maximal pressure, atm | 16±2 | 16±2 | .64 |
| Maximal balloon | 3 [2.75-3.25] | 3 [2.75-3.25] | .44a |
| Balloon-to-artery ratio | 1.16±0.2 | 1.24±0.2 | .15 |
| Crossover | 0 (0) | 0 (0) | 1b |
| Angiographic success | 23 (100) | 41 (100) | 1b |
| Acute angiographic results | |||
| Minimal lumen diameter, mm | 2.09±0.5 | 2.04±0.4 | .71 |
| Stenosis (% of lumen diameter) | 17±11 | 17±12 | .86 |
| Acute gain, mm | 1.31±0.6 | 1.31±0.5 | .99 |
EES, everolimus-eluting stent; NA, neoatherosclerosis; PCB, paclitaxel coated balloon.
The data are expressed as No. (%), mean±standard deviation or median [interquartile range].
Angiographic follow-up at 6 to 9 months showed no differences in restenosis [5 (24%) vs 6 (15%) P=.49], minimum lumen diameter (1.79±0.7 vs 1.94±0.6mm, P=.41) or late loss (0.33±0.7 vs 0.15±0.5, P=.34) in patients with and without neoatherosclerosis, respectively.
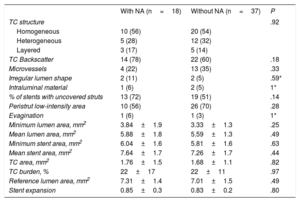
Follow-up OCT confirmed the absence of differences in quantitative parameters (minimum and mean lumen area, minimum and mean stent area, stent expansion, tissue coverage area) between the 2 groups. The characteristics of tissue coverage at follow-up were also similar in both groups with no differences in strut coverage or presence of evaginations (table 4).
Optical coherence tomography follow-up
| With NA (n=18) | Without NA (n=37) | P | |
|---|---|---|---|
| TC structure | .92 | ||
| Homogeneous | 10 (56) | 20 (54) | |
| Heterogeneous | 5 (28) | 12 (32) | |
| Layered | 3 (17) | 5 (14) | |
| TC Backscatter | 14 (78) | 22 (60) | .18 |
| Microvessels | 4 (22) | 13 (35) | .33 |
| Irregular lumen shape | 2 (11) | 2 (5) | .59* |
| Intraluminal material | 1 (6) | 2 (5) | 1* |
| % of stents with uncovered struts | 13 (72) | 19 (51) | .14 |
| Peristrut low-intensity area | 10 (56) | 26 (70) | .28 |
| Evagination | 1 (6) | 1 (3) | 1* |
| Minimum lumen area, mm2 | 3.84±1.9 | 3.33±1.3 | .25 |
| Mean lumen area, mm2 | 5.88±1.8 | 5.59±1.3 | .49 |
| Minimum stent area, mm2 | 6.04±1.6 | 5.81±1.6 | .63 |
| Mean stent area, mm2 | 7.64±1.7 | 7.26±1.7 | .44 |
| TC area, mm2 | 1.76±1.5 | 1.68±1.1 | .82 |
| TC burden, % | 22±17 | 22±11 | .97 |
| Reference lumen area, mm2 | 7.31±1.4 | 7.01±1.5 | .49 |
| Stent expansion | 0.85±0.3 | 0.83±0.2 | .80 |
NA, neoatherosclerosis; TC, tissue coverage.
The data are expressed as No. (%) or mean±standard deviation.
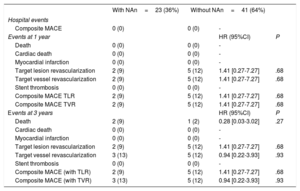
Clinical follow-up at 1 and 3 years was obtained in 64 (100%) patients. Table 5 and figure 4 show the rates of major adverse cardiovascular events (MACE) and their individual components (cardiac death, myocardial infarction, target lesion revascularization, target vessel revascularization) in patients with or without neoatherosclerosis treated with PCB or EES.
Major adverse clinical events
| With NAn=23 (36%) | Without NAn=41 (64%) | |||
|---|---|---|---|---|
| Hospital events | ||||
| Composite MACE | 0 (0) | 0 (0) | - | |
| Events at 1 year | HR (95%CI) | P | ||
| Death | 0 (0) | 0 (0) | - | |
| Cardiac death | 0 (0) | 0 (0) | - | |
| Myocardial infarction | 0 (0) | 0 (0) | - | |
| Target lesion revascularization | 2 (9) | 5 (12) | 1.41 [0.27-7.27] | .68 |
| Target vessel revascularization | 2 (9) | 5 (12) | 1.41 [0.27-7.27] | .68 |
| Stent thrombosis | 0 (0) | 0 (0) | - | |
| Composite MACE TLR | 2 (9) | 5 (12) | 1.41 [0.27-7.27] | .68 |
| Composite MACE TVR | 2 (9) | 5 (12) | 1.41 [0.27-7.27] | .68 |
| Events at 3 years | HR (95%CI) | P | ||
| Death | 2 (9) | 1 (2) | 0.28 [0.03-3.02] | .27 |
| Cardiac death | 0 (0) | 0 (0) | - | |
| Myocardial infarction | 0 (0) | 0 (0) | - | |
| Target lesion revascularization | 2 (9) | 5 (12) | 1.41 [0.27-7.27] | .68 |
| Target vessel revascularization | 3 (13) | 5 (12) | 0.94 [0.22-3.93] | .93 |
| Stent thrombosis | 0 (0) | 0 (0) | - | |
| Composite MACE (with TLR) | 2 (9) | 5 (12) | 1.41 [0.27-7.27] | .68 |
| Composite MACE (with TVR) | 3 (13) | 5 (12) | 0.94 [0.22-3.93] | .93 |
95%CI, 95% confidence interval; HR, hazard ratio (events at follow-up); MACE TLR, major adverse cardiac events (cardiac death, myocardial infarction, target lesion revascularization); MACE TVR, major adverse cardiac events (cardiac death, myocardial infarction, target vessel revascularization); NA, neoatherosclerosis.
P values from Cox analysis.
The data are expressed as No. (%) or median [interquartile range].
A: survival curve in patients with and without neoatherosclerosis. B: survival curve in patients with and without neoatherosclerosis in the PCB group. C: survival curve in patients with and without neoatherosclerosis in the EES group. EES, everolimus-eluting stent; FU, follow-up; MI, myocardial infarction; NA, neoatherosclerosis; PCB, paclitaxel-coated balloon; Pts, patients; TVR, target vessel revascularization.
Interestingly, in patients with neoatherosclerosis treated with PCB, the restenosis rate at follow-up was numerically lower (nonsignificant) (1 of 9 [11%]) than in lesions without neoatherosclerosis (4 of 20 [20%] P=1). Conversely, in patients treated with EES there was a tendency toward a higher restenosis rate (nonsignificant) in the neoatherosclerosis group [4 of 12 (33%) vs 2 of 21 (10%) P=.16]. With regards to MACE, in this limited population, similar rates were observed in the PCB group between patients with and without neoatherosclerosis. Comparable results for clinical outcomes were observed in patients with and without neoatherosclerosis treated with EES.
Of the 23 patients with neoatherosclerosis, 10 were treated with PCB and 13 with EES. The restenosis rate was 1 of 9 (11%) in PCB vs 4 of 12 (33%) in EES (P=.34). MACE at 1- and 3-year follow-up was low in this population with no cases of death or myocardial infarction. Only 1 target lesion revascularization occurred in each arm at 1 year. In this small group, MACE at the 1- and 3-year follow-up was similar in patients with neoatherosclerosis treated with PCB and EES.
DISCUSSIONIn this OCT-based subanalysis of the RIBS IV and V trials we found that: a) a substantial number of ISR lesions undergoing repeat revascularization show neoatherosclerosis; b) in this limited study population, the presence of neoatherosclerosis did not seem to influence acute and long-term re-PCI outcomes; and c) the current study does not allow the evaluation of differences in outcomes between EES and PCB for the treatment of ISR with neoatherosclerosis but might suggest that both treatment options could be effective and safe. This is of relevance as currently both PCB and EES are recommended by clinical practice guidelines for the treatment of patients with ISR.
Other findings are as follows: a) ISR with neoatherosclerosis more frequently had an unstable clinical presentation. b) This correlated with the more frequent presence of OCT findings suggestive of instability such as the presence of intraluminal material, irregular lumen, and macrophages.
Influence of neoatherosclerosis on repeat PCI outcomesNeoatherosclerosis, defined as the presence of atherosclerotic plaque in intrastent neointimal tissue, has been shown as a cause of stent failure (both restenosis and stent thrombosis).9,10 Duration of implant, DES use, chronic kidney disease and high low-density lipoprotein levels have been described as predictors of this entity.11–16 The prevalence of neoatherosclerosis reported in the literature varies depending on the stent type, the indication for OCT at follow-up, and implant duration. Thirty-six percent of our cases of ISR showed signs of neoatherosclerosis, a similar prevalence to that described in previous reports.
Previous retrospective studies in patients undergoing OCT evaluation at follow-up after stent implantation have demonstrated that those with a neointima containing neoatherosclerosis had a worse prognosis with a higher rate of events and revascularizations.17–19 However, these studies did not provide information on the impact of neoatherosclerosis in clinically relevant ISR undergoing new revascularization.
This is the first study investigating the influence of OCT-derived neoatherosclerosis on the long-term outcomes of patients with ISR requiring repeat PCI (with symptoms or ischemia). The strength of the observations stem from the prospective nature and strong methodology used in the randomized RIBS IV and V trials. In this regard, it is reassuring that the presence of neoatherosclerosis did not seem to influence the acute and long-term prognosis of these patients. This is in agreement with the angiographic follow-up (with no differences in minimum lumen diameter or late loss between groups) and the OCT results at follow-up, which demonstrated a similar treatment response in patients with and without neoatherosclerosis, with no differences in the amount or optical characteristics of tissue coverage.
Interestingly, the present study suggests that neoatherosclerosis might be safely and effectively treated with PCB with similar acute and long-term outcomes to those treated with EES in our population. The presence of an underlying scaffold might explain the differences in the response of neoatherosclerosis and native atherosclerosis to PCB treatment.
Our findings are in agreement with a previous study by Tada et al.20 evaluating the influence of OCT ISR patterns (homogeneous, heterogenous or layered neointima) on mid-term results of re-PCI with plain balloon angioplasty PCB or DES. The most frequent optical pattern in ISR with neoatherosclerosis in our population was heterogeneous, as has been also shown in a histological study assessing neointimal characteristics after DES implantation.21 Tada et al.20 did not find differences in ISR, target lesion revascularization and late loss at 6-8 months between patients with heterogeneous ISR pattern treated with PCB or DES, a result concordant with our data.
In the group treated with EES there was a nonsignificant trend toward a higher restenosis rate in patients with neoatherosclerosis while in the group treated with PCB the rate of restenosis was numerically lower in patients with neoatherosclerosis. The limited sample size does not allow more definitive conclusions. Further studies are needed to evaluate a possible differential response to the different treatment approaches.
Neoatherosclerosis and clinical presentationIn our study, patients with ISR and neoatherosclerosis more frequently presented as unstable angina while patients without neoatherosclerosis had a more stable presentation. This is in agreement with a previous DES-ISR study by Kang et al.6 showing that patients with unstable (vs stable) angina more often had a thin cap fibroatheroma-containing neointima or neointima rupture. Presentation as acute coronary syndrome was also more frequent in lesions with> 50% neointimal cross sectional area when they had neoatherosclerosis. The rupture of a lipid containing neointima with subsequent thrombus formation can generate an unstable clinical presentation and has been proposed to explain the link between ISR and late stent thrombosis.22 Our results demonstrate a higher frequency of irregular lumen and intraluminal material (suggestive of thrombus) in patients with neoatherosclerosis. Macrophages, another marker of plaque instability, were also more frequent in patients with neoatherosclerosis.
Relation between neoatherosclerosis and PSLIAIn our study, PSLIA were less often observed in patients with neoatherosclerosis. This OCT finding has been related in histology with the presence of fibrinoid and proteoglicans, as well as with neovascularization and peristrut inflammation.23 Several studies have shown a correlation between PSLIA and neointimal thickness and an inverse relation with time from stent implantation.24 Therefore, PSLIA seems to be a phenomenon related with vessel healing after stent implantation while neoatherosclerosis consists of new atherosclerosis development inside the stent. According to our findings, current evidence does not show an association between these 2 phenomena, even though inflammation could play a role in both. Concordantly with previous reports, patients without neoatherosclerosis in our study (who more frequently had PSLIA) had a shorter time from stent implantation and a higher (though nonsignificant) amount of restenotic tissue.
LimitationsThe main limitation of this study is its small sample size. The OCT substudy of the RIBS IV and VI trial was only conducted in some of the participating centers, which limited patient inclusion. However, there were no differences in the characteristics of the patients included in the OCT substudy and those who were not. While the results of the present study regarding neoatherosclerosis response to treatment with PCB or EES do not allow definitive conclusions to be drawn, they can be hypothesis generating. A randomized trial of the 2 strategies for the treatment of patients with ISR and neoatherosclerosis would be needed to confirm the results. Based on our results, we have estimated that the sample size required for this randomized study would be 376 patients in each group, making it difficult to perform, given the limited number of patients with ISR, of whom only the patients with neoatherosclerosis could be selected. Pathological correlations have shown the potential overestimation in the diagnosis of neoatherosclerosis with OCT, especially regarding thin cap fibroatheroma detection.23 Neoatherosclerosis is a heterogeneous entity in itself, including calcium and lipid formation inside the neointima that might potentially respond in a different manner to treatment with PCB or DES. The limited numbers in our study do not allow conclusions in this regard.
CONCLUSIONSOCT has provided new scope in the evaluation of ISR, allowing the identification of neoatherosclerosis in vivo and revealing a variety of patterns and mechanisms that might potentially require different treatment strategies. In this limited study population, OCT-defined neoatherosclerosis did not seem to influence acute and long-term outcomes in patients randomized to PCB or EES for ISR. In addition, our findings could suggest that ISR with neoatherosclerosis might be treated with both PCB and EES. The present results are only hypothesis generating and further studies are needed to compare the 2 treatment strategies in this type of ISR.
FUNDINGThe RIBS trials were an investigator-driven initiative organized under the auspices of the Working Group on Interventional Cardiology of the Spanish Society of Cardiology. The sudy promotor was the Fundación Interhospitalaria para la Investigación Cardiovascular (FIC). B. Braun Surgical and Abbott Vascular provided unrestricted research grants.
CONFLICTS OF INTERESTN. Gonzalo has received speaker honoraria for educational activities from Abbott Medical. The other authors have no disclosures to report.
- -
Neoatherosclerosis, defined as the presence of atherosclerotic plaque in intrastent neointimal tissue, has been shown as a cause of in-stent restenosis. OCT is a high-resolution intracoronary imaging technique that allows the identification of neoatherosclerosis in vivo. Little is known about whether neoatherosclerosis influences the long-term outcomes in patients with ISR treated with PCI, or whether PCB or EES are equally effective in treating ISR with neoatherosclerosis.
- -
This is the first study investigating the influence of neoatherosclerosis on prognosis and treatment response in patients treated with EES or PCB for ISR.
- -
In our limited study population, OCT-defined neoatherosclerosis did not influence acute and long-term outcomes in patients treated for ISR.
- -
Our findings may suggest that ISR with neoatherosclerosis could be treated with PCB or everolimus-eluting stents.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.03.005