Keywords
INTRODUCTION
Data from Spain's national statistics office for 2007 confirms that cardiovascular diseases continue to be the leading cause of mortality (33.7% of all deaths). Ischemic heart disease is the primary cause of death in men and cerebrovascular disease in women.1 However, thanks to the appearance of new treatments, mortality in patients with acute coronary syndrome (ACS) has fallen in recent years. Ischemic heart disease frequently presents as non-ST segment elevation acute coronary syndrome (NSTEACS). Annual incidence of hospital admissions is 3 per 1000 inhabitants, with 5% in-hospital mortality rising to 13% at 6 months.2,3
In Spain, NSTEACS management guidelines were first published in 2000.4 An update appeared in 20025 and the European Society of Cardiology published the most recent guidelines to daily clinical practice in the diagnosis and treatment of NSTEACS in 2007.6 They remain in force. These guidelines are intended to encourage the standardized management of patients with NSTEACS. The recent guidelines are clear on the management and treatment of patients with NSTEACS. They particularly emphasize the importance of risk-stratification for subsequent decision-making in management and choice of revascularization strategy. As a function of risk level, the guidelines recommend an invasive strategy in moderate-high risk patients who should undergo coronary angiography at the first 72 hours as first choice treatment. This contrasts with the more conservative approach recommended for low-risk patients. Two recent NSTEACS registries describe general management of the condition in Spain7,8: the MASCARA (Updated Management of Acute Coronary Syndrome Registry 2006) study8 describes a more substantial adaptation of clinical practice guidelines than the DESCARTES (Description of the State of Acute Coronary Syndromes in a Timed Spanish Registry) study, constructed 4 years earlier.7
However, daily clinical practice in hospitals must allow for variables that guidelines do not evaluate (logistic or structural limitations in the centers, lack of catheterization laboratories and transfer problems). These can condition the way patients are treated and influence prognosis. Moreover, care afforded to patients with ischemic heart disease (including those with ACS) differs from one part of Spain to another.9 Factors that could influence variations in the use of diagnostic and therapeutic procedures include differences in the availability of resources, application of clinical practice guidelines, and the level of implantation and standardization of protocols for patient referral or the almost complete absence of these protocols.10 These discrepancies could have significant repercussions on morbidity and mortality due to ACS. Hence, identifying them would standardize patient management to a greater extent.
The objective of the present study is to analyze differences in the management of consecutive patients with NSTEACS admitted to Spanish hospitals with catheterization laboratories (ie, tertiary-care hospitals, THs) and hospitals without laboratories (ie, secondary-care hospitals, SHs). In addition, we try to determine differences in in-hospital medical treatment and in the use of invasive procedures (cardiac catheterization and revascularization) and their possible influence on short- and mid-term prognosis.
METHODS
The principle objective of our voluntary, multicenter, retrospective study of guidelines and ACS (GYSCA) is to analyze the application of clinical practice guidelines in NSTEACS management. We enrolled all consecutive patients diagnosed with NSTEACS and admitted to the participating Spanish hospitals over a period of 4 months (February 18 to June 15, 2007). The only inclusion criterion was admission with a diagnosis of NSTEACS: chest pain typical of ischemia and unstable, accompanied or not by electrocardiographic and/or enzyme markers. In each hospital, the research team physician(s) obtained data on all admissions, whether to regular wards or coronary or intensive care units, having previously obtained informed written consent. We excluded patients attended in Emergency Room with a final diagnosis of NSTEACS who were not admitted to participating centers (patients who were discharged or transferred to other hospitals).
Our registry was approved by the regional clinical research ethics committee of the healthcare service for the Spanish autonomous region of Asturias on December 11, 2006.
Fifteen hospitals participated in the study: 6 THs (with catheterization laboratories) and 9 SHs (without laboratories). The SHs transferred patients to the 6 THs for catheterization. The only criterion for catheterization was the judgment of the attending physician in each hospital. No a priori indications were established. A list of participating centers and researchers appears at the end of the present paper.
We created a centralized database to which information was added online via a webpage. We recorded >170 variables per patient drawn from case history, physical examination, clinical, analytical, electrocardiographic and other signs and symptoms related with therapeutic management (at admission, discharge, and during follow-up) and revascularization strategies (in-hospital and during follow-up). Moreover, following admission, we recorded any transfer of patients from one hospital unit to another, or from one center to another, for catheterization or heart surgery. The research team physician at the hospital of initial admission was responsible for data collection and patient follow-up. Thus, patients were always assigned to the center to which they had initially been admitted.
Researchers had to include data on most of the variables to guarantee the high quality of data collected. Three-month and 1-year follow-up was conducted in the clinic or by telephone.
In the follow-up, we studied cardiac-cause and overall mortality, readmissions for NSTEACS or ST-segment elevation acute coronary syndrome (STEACS), revascularization, and the combined outcome of major adverse cardiac events (MACE), which included cardiac death, admission for ACS or revascularization. Programmed percutaneous or surgical revascularization following admission was not considered an event.
Statistical Analysis
Discrete variables are presented as frequencies (percentages). Comparison of discrete variables was with χ2. Numerical variables are presented as mean (SD). Comparison of the 2 groups' results for continuous variables was with Student t test for non-paired data. We constructed Kaplan-Meier survival curves and compared these with the log-rank test. We performed Cox regression analysis to determine predictors of MACE at 1 year. As well as hospital type and variables of undoubted clinical interest, we included potential confounding factors recording P<.15 in both univariate analysis comparing hospital type and in univariate analysis of patients with and without MACE during follow-up. Values of P were 2-tailed. Statistical significance was established at P<.05. Statistical power >80% was guaranteed. Statistical analysis was with SPSS 12.0.
RESULTS
We enrolled 1133 patients in the registry: 599 (52.9%) in THs and 534 (47.1%) in SHs. Baseline characteristics of patients belonging to the 2 groups are in Table 1. Patients admitted to SHs were older (67.9 [11.6] vs 70.3 [12.2] years; P<.01), had greater incidence of dyslipidemia and a lower percentage of positive myocardial damage markers at admission (57.5% vs 71.8%; P<.01).
We stratified patient risk with the TIMI11 risk classification and the GRACE12 scales, as recommended in the latest European Society of Cardiology guidelines. Both analyze clinical, analytical and electrocardiographic (ECG) variables. Patients stratified by risk on the two scales were similar in both populations (Table 1).
One noteworthy difference in the management of the 2 populations is the clinical services where they are treated: 21% of SH patients were admitted to internal medicine, and higher percentages of TH patients were admitted to specialized units (intensive care or coronary units): 41.4% vs 24.9% (P<0.01) (Table 2).
In-hospital treatment more closely matched therapeutic management guidelines in THs than in SHs (Figure 1A): antiplatelet therapy, anticoagulants, angiotensin converting enzyme (ACE) inhibitors, beta-blockers, and statins were used more frequently in THs. Neither THs nor SHs made much use of glycoprotein IIb/IIIa receptor inhibitors. Patients in THs underwent catheterization more often (70% vs 49%; P<.01), which led to more in-hospital revascularization procedures (Table 2). Percutaneous revascularization was the procedure of choice in 85% of patients. Incidence of coronary disease found through catheterization was similar in both populations, with similar percentages of coronary arteries without significant lesions in both hospital types (THs, 13%; SHs, 17.9%; P=.1).
Figure 1. A: treatment during hospitalization. B: treatment on discharge. AAS indicates acetylsalicylic acid; ACEI, angiotensin converting enzyme inhibitors; Anti-GPIIb/IIIa, glycoprotein IIb/IIIa receptor inhibitors; BB, beta-blockers; CaA, calcium channel antagonists; LMWH, low molecular weight heparin.
In-hospital patient clinical course was similar in both groups, with mortality of 3.5% in THs and 3.7% in SHs. Minor hemorrhage was more frequent in SH patients (4% vs 0.7%; P<.01).
Treatment administered at discharge was closer to current recommendations in THs, with greater use of aspirin, beta blockers, ACE inhibitors and statins (Figure 1B).
Follow-up
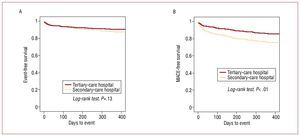
At 1 year, complete follow-up data was available for 95.5% of the series. Events during follow-up are in Table 3. Cardiac mortality was similar in both groups (9.1% vs 9.4%; P=.45). During the 1-year follow-up, admission for NSTEACS was 5 times greater in SHs than in THs (12.8% vs 2.3%; P<.01). Survival curves were similar in both groups (Figure 2A) (log-rank test; P=.13) but analysis of MACE revealed a significantly better clinical course in patients admitted to THs (Figure 2B: log-rank test; P<.01; and Figure 1C: log-rank test, P<.01). Univariate analysis of patients with and without MACE during follow-up is in Table 4.
Figure 2. A: Kaplan-Meier survival curve for any-cause death. B: Kaplan-Meier curve for MACE-free survival (cardiac death, NSTEACS or revascularization).
Cox regression analysis showed MACE was predicted by hospital-of-admission type (TH or SH), dyslipidemia, ST-segment depression in ECG on admission, presence of positive troponins, and absence of revascularization during hospitalization (Table 5).
DISCUSSION
The GYSCA registry describes how patients diagnosed with NSTEACS and admitted to hospitals with catheterization laboratories undergo more invasive treatment and are administered drug regimes which more closely match therapeutic recommendations of cardiological societies' NSTEACS management guidelines than patients admitted to hospitals without laboratories. Although in-hospital prognosis is similar, SH patients present a higher frequency of readmission for NSTEACS per year than do TH admissions.
The GYSCA registry provides information on the clinical profile, management and prognosis of patients hospitalized for NSTEACS in Spanish centers. It also reports on the level of fulfillment of the latest European guideline recommendations in a current population of 15 Spanish hospitals. The type of registry enables us to establish a distinction in management between hospitals with and hospitals without catheterization laboratories.
We find that NSTEACS patient management in THs is more invasive, a higher percentage of patients undergo in-hospital revascularization procedures, and drug treatment is better than in SHs. Use of antiplatelet agents (aspirin, clopidogrel, and glycoprotein IIb/IIIa receptor inhibitors), beta-blockers, ACE inhibitors, and statins is greater in TH patients.
This improved in-hospital management does not appear to have an initial impact during hospitalization, and the complications are similar, as is mortality. This was to be expected since higher-risk patients or those for whom medical treatment fails are referred for catheterization. The distribution of events that our registry presents is a constant in all studies that analyze the impact of invasive treatment versus a more conservative approach in patients with NSTEACS. Initially, the rate of ischemic events does not significantly differ between the two strategies (at first, the invasive strategy is penalized by a higher rate of procedure-associated events). In mid-term follow-up, the benefits of revascularization determine a significant reduction in the rate of events in patients who initially received the more invasive treatment. Significantly, TH patients present a better prognosis at 1 year as evidenced by the fall in readmissions for NSTEACS. Moreover, incidence of MACE in THs is almost half that in SHs (13.4% vs 23.5%; P<.01).
Are the patient populations admitted to the 2 hospital types in fact comparable? As Table 1 indicates, they are quite alike and the prevalence of risk factors is similar. In SHs, the population is slightly older but in THs presence of positive troponins at admission is higher. If we stratify both populations as European guidelines recommend,6 we find that patients at low-, medium- and high-risk on both the GRACE and TIMI scales are identical.
To avoid possible confounding variables, we conducted multivariate analysis to determine predictors of MACE in 1-year prognosis in our patients. As well as non-modifiable risk factors (dyslipidemia) and variables indicating more serious symptoms (ST-segment depression or positive troponins on admission), admission to a hospital without a catheterization laboratory was a variable that predicted MACE independently of the benefits of revascularization.
Although the existence of confounding variables which are difficult to identify could influence results, our findings are supported in a number of ways:
1. Logically, the presence of a catheterization laboratory in situ facilitates the more invasive management of patients: 70.8% of TH patients undergo catheterization versus only 49.2% of SH patients (P<.01). This leads to a higher number of TH patients undergoing revascularization during hospitalization (43.1% vs 29.7%; P<.01), which could condition an improved prognosis in patients with NSTEACS.13 Moreover, we cannot ignore how complex the transfer of older patients with high comorbidity to hospitals with a catheterization laboratory can be. Limited opportunities for cardiac catheterization procedures often condition their performance in younger patients with NSTEACS and their indication is restricted in the subgroups of older patients with comorbidities who, paradoxically, are at greater risk.14,15
2. A much higher percentage of TH patients are hospitalized in specialized units (coronary or intensive care) and a not-inconsiderable percentage of SH patients are treated in internal medicine services (21% according to the GYSCA registry), where knowledge of indications for catheterization and about the management of these patients may be inferior. This may influence prognosis.
3. Less-than-optimal drug regimes administered in SHs both on admission and at discharge may also contribute to this worse prognosis.
The better prognosis of patients treated for NSTEACS in THs should give rise to a series of actions that would correct the difference observed in the management of SH patients. Essentially, to ensure a more direct relationship between the physicians involved in managing these patients in SHs and the physicians in catheterization laboratories, common protocols of action are needed that would facilitate to the utmost the admission of patients referred by other hospitals. Furthermore, training policies should be implemented, especially for non-cardiologists, which favor the application of guideline recommendations to optimize drug treatment. These measures should help minimize the differences in the clinical course of patients admitted for a condition for which one factor that can influence prognosis is hospital-of-admission type.
Limitations of our Study
The fact that our information is generated by a registry should be considered a limitation. Registries are thought to be highly representative of daily clinical practice and they more adequately report rates of clinical events than do control studies. They also include non-ideal patients who are at higher risk although they do enable us to learn whether guidelines are applied adequately. Secondly, the hospitals in our study were not selected at random as this was a voluntary registry. In each hospital, one or two researchers recruited all patients consecutively admitted to their own or other units. We must recognize this may have influenced patient management. A further limitation is the fact that evaluation of the impact of a specific treatment (more invasive strategy) through a registry may be incorrect due to the influence of confounding variables that are not evaluated. Furthermore, an inevitable risk of bias exists in the selection and in the prognostic potential. Nonetheless, the data in our study do coincide with the conclusions of several randomized studies. As mentioned earlier, our only inclusion criterion was the clinical diagnosis of NSTEACS (unstable ischemic chest pain with or without electrocardiographic and/or enzyme markers) which means some patients included may not really have had ACS. One final limitation is the absence of an external quality control mechanism. While the webpage data collection system was designed to ensure the inclusion of >95% of the variables and the voluntary nature of the participating researchers facilitated the inclusion of all consecutive patient admissions for NSTEACS over a short period of 4 months, the absence of an external mechanism means the optimal quality of data is not guaranteed.
CONCLUSIONS
The GYSCA registry describes how patients admitted for NSTEACS to hospitals without a catheterization laboratory receive less invasive treatment and are administered drug regimens on admission and at discharge that less closely match guideline recommendations. Together with those factors known to predict prognosis, hospital type can have an additional impact on clinical course.
PARTICIPATING HOSPITALS AND RESEARCHERS
Tertiary-Care Hospitals
Hospital Central de Asturias: César Morís de la Tassa, José Manuel García, and María Martín.
Hospital de Bellvitge, L'Hospitalet de Llobregat, Barcelona: Àngel Cequier Fillat, José Luis Ferreiro, Laura Pinilla, and Joan Antoni Gómez Hospital.
Hospital de León: Felipe Fernández, Emilio Malpierca, Armando Pérez de Prado, and Carlos Cuellas.
Hospital General Universitario de Alicante: Juan Miguel Ruiz-Nodar and Sergio Abán Alique.
Hospital Son Dureta, Palma de Mallorca: Armando Bethencourt and Gaspar Melis.
Hospital Marqués de Valdecilla, Santander: Virginia Burgos Palacios and José María de la Torre.
Secondary-Care Hospitals
Hospital de Villajoyosa, Alicante: Teresa Lozano Palencia and María Teresa Enguix.
Hospital San Agustín, Avilés, Asturias: Inés Möller and María Rengel.
Hospital Son Llàtzer, Palma de Mallorca: Joan Torres Marqués.
Hospital de Elda, Alicante: Francisco González Llopis.
Hospital de Viladecans, Barcelona: Pere Álvarez García.
Hospital de Manacor, Baleares: Bernardo García de la Villa Redondo.
Hospital de Sierrallana, Torrelavega, Cantabria: Manuel Jesús Zarauza Navarro.
Hospital Valle del Nalón, Langreo, Asturias: Águeda García Rodríguez.
ABBREVIATIONS
ACS: acute coronary syndrome
GYSCA: guidelines and acute coronary syndrome registry
MACE: major adverse cardiovascular events
NSTEACS: non-ST segment elevation acute coronary syndrome
STEACS: ST-segment elevation acute coronary syndrome
The GYSCA registry received a grant from Merck Sharp & Dhome.
Correspondence: Dr. J.M. Ruiz-Nodar.
Servicio de Cardiología. Hospital General Universitario de Alicante. Maestro Alonso, s/n. 03010 Alicante. España.
E-mail: ruiz_jmi@gva.es
Received February 13, 2009.
Accepted for publication October 14, 2009.