INTRODUCTION
Hyperglycemia may be observed at admission in patients with acute myocardial infarction, independently of prior history of diabetes, and is associated with an increase in mortality.1,2 The increased mortality may be explained by larger infarct size and a higher rate of heart failure and cardiogenic shock in that population.3-5
In some patients, elevated glucose levels may simply be a marker of preexisting but as yet undetected disease, type 2 diabetes or glucose intolerance,6 leading to an increase in lipolysis and an excess of circulating free fatty acids, more severe myocardial damage, and even more severe coronary disease.5,7-10
Little is known about the role of hyperglycemia at admission in patients with acute coronary syndrome, since only a few studies have analyzed this marker in the population of patients with non-ST-segment elevation myocardial infarction and unstable angina, and in those that have, the follow-up period has been limited. Even less is known in Latin American populations, as diagnosis of infarction is often delayed and glucose levels may be just another simple marker in this situation. This study addressed the hypothesis that patients with acute coronary syndrome and increased glucose levels show worse long-term outcome.
The aim of the study was to analyze the long-term prognostic value of glucose concentration at admission in patients with acute coronary syndrome.
METHODS
Population
A prospective observational study was performed in a population of 565 consecutive patients admitted between December 1997 and December 2001 to the Coronary Care Unit of the Instituto de Cardiología, Corrientes, Argentina, with a diagnosis of acute coronary syndrome within 24 hours of the onset of symptoms.
Inclusion Criteria
1. Acute coronary syndrome, defined as typical precordial pain for at least 20 minutes along with the presence of 1 or more of the following conditions: new or presumably new ST-segment deviation, altered enzyme levels (creatine kinase MB isoenzyme [CK-MB] level above the reference limit in 2 or more samples obtained within a period of more than 6 hours and/or troponin T≥0.02 ng/mL).
2. Acute myocardial infarction, defined as the presence of at least 2 of the following 3 conditions: a) appearance of new Q waves, b) CK-MB levels above the reference limit, and c) precordial pain lasting at least 30 minutes. According to the above criteria, the remaining patients were considered to have unstable angina.
Exclusion Criteria
Admission more then 24 hours after onset of symptoms, cardiogenic shock, acute pulmonary edema, suspected myocarditis, pulmonary thromboembolism, congenital disease, dilated cardiomyopathy, cardiac hypertrophy, valve disease or pericardial disease, and difficulty in completion of follow-up.
Study Protocol
The study protocol was approved by the Department of Research and Teaching of our institution. Patients were informed regarding inclusion in the study and provided signed consent.
All patients were admitted to the coronary care unit, where a full medical history was taken along with a physical examination, 12-lead electrocardiogram (ECG) at admission and 2 hours after reperfusion therapy in those patients in whom ST-segment elevation was observed, chest radiograph at admission and 24 hours later, analysis of enzyme concentrations (CK, CK-MB) at admission, 2 hours after admission or reperfusion therapy, at 6, 12, and 24 hours, and then every 24 hours until enzyme concentrations returned to normal, and concentrations of troponin T as a marker of myocardial necrosis at admission.
Final diagnosis was acute myocardial infarction (AMI) in 56% of patients (n=317) and unstable angina in 44% (n=248).
Definitions
- Hyperglycemia: The population was split into 2 groups according to the cut point obtained from the receiver operating characteristics (ROC) curve to predict mortality. Group 1 and group 2 contained patients with glucose concentrations of ≥128 mg/dL, and <128 mg/dL, respectively.
- Diabetes: presence of fasting glucose concentrations >126 mg/dL, diagnosed prior to admission.
- Q-wave infarction: defined after 24 hours according to the presence of new Q waves in the V1-V3 leads or the development of Q waves ≥0.03 seconds in leads I, II, aVL, aVF, V4, V5, and V6.11
- Non-Q-wave infarction: considered in the absence of new Q waves in the ECG 24 hours after admission. It should be noted that the old definition of infarction was used.
- Heart failure: defined by the presence of typical symptoms, rales, S3 gallop, and evidence of pulmonary congestion in the chest radiograph (flow redistribution, interstitial edema, and/or alveolar edema), necessitating the use of intravenous diuretics, vasodilators, and/or inotropic drugs.
- Mortality: all-cause death was calculated at the end of follow-up.
Follow-Up and Endpoints
The follow-up period lasted until 6 months after inclusion of the last patient. Follow-up was performed using hospital medical records and patients were assessed in the ischemic heart disease clinic (82%) or by their cardiologist (13%); the remaining 5% were contacted by telephone or through their primary care physician. The mean follow-up period was 42 (9) months.
Hospital Treatment
Following admission, most patients received oral aspirin (100-325 mg/day). Patients with ST-segment elevation admitted in the 12 hours following onset of symptoms were treated by primary angioplasty or with thrombolytic drugs. Intravenous nitroglycerin was administered in 92% of patients and, in the absence of contraindications, intravenous or oral β-blockers and angiotensin converting enzyme inhibitors (ACEI) were also prescribed.
Statistical Analysis
A ROC curve was constructed to determine the best cut point for glucose concentration in the prediction of death during follow-up; using this method, hyperglycemia was classified as a glucose concentration ≥128 mg/dL. The characteristics of the patients with glucose levels greater than or equal to and below the cutoff were compared by χ&SUP2; test for qualitative variables and the results were expressed as percentages. Quantitative variables were expressed as means (SD) and analyzed using the Student t test for normally distributed variables and the Mann-Whitney U test for variables that were not normally distributed. Two Cox proportional hazards models were constructed to identify independent predictors of mortality during follow-up. The following variables, which were significant in the univariate analysis, were included in both models: male sex, systolic blood pressure, heart rate, and troponin-T level at admission, diagnosis of infarction, and heart failure during hospital stay, and in addition, glucose concentration was included as a qualitative variable with a cutoff of ≥128 mg/dL (model 1) and as a quantitative variable with increments of 10 mg/dL (model 2). Differences were considered statistically significant when P<.05. Kaplan-Meier survival analysis was applied and log-rank tests were used to compare curves. Analysis was performed using the statistical package SPSS 10.0 for Windows (SPSS Inc, Chicago, Illinois, USA). Eleven patients were lost to follow-up.
RESULTS
Patient Characteristics
The general characteristics of the study population are shown in Table 1. The mean glucose level at admission was 143 (77) mg/dL. A ROC curve was constructed to determine the best cut point for prediction of mortality during follow-up. The area under the curve was 0.67 (95% confidence interval [CI], 0.60-0.75) (Figure 1). A total of 208 patients (36.8%) had glucose levels ≥128 mg/dL at admission (group 1); the remaining patients made up group 2.
Figure 1. Receiver operating characteristic (ROC) curve for prediction of death at 4-year follow-up. ROC curve for glucose level (area below the curve, 0.67; 95% confidence interval, 0.60-0.75); cut point, 128 mg/dL; sensitivity, 85%; specificity, 62%.
The mean age was similar in both groups (63.1 [11.5] vs 61.2 [12.5] years, P=.76). Among the demographic characteristics of the patients at admission, a higher proportion of diabetes and hypertension, and a larger number of women were found in group 1 (Table 1).
When clinical characteristics were analyzed, it was observed that group 1 had more hypertension and tachycardia at admission, with a larger number of affected coronary vessels, higher concentrations of markers of myocardial necrosis, and a lower left ventricular ejection fraction (Tables 2 and 3).
Treatment
In this series of patients included in the 24 hours following onset of symptoms, reperfusion therapy with thrombolytic drugs was used to a similar extent in both groups. However, there was greater use of primary angioplasty in patients with infarction in the hyperglycemic group. The patients in group 1 were more often prescribed ACEI, while β-blockers were used more often in group 2 (Table 4).
Predictors of Mortality and Analysis of Survival
Mortality during follow-up was 9.7%. Mortality was 3 times higher in patients with glucose levels ≥128 mg/dL (16.1% vs 4.7%; P<.0001). Heart failure was developed during the period of hospital admission in 12.7% of the population, with a higher proportion in group 1 (21.4% vs 6%, P<.001) (Table 5).
The following variables were independent predictors of mortality at admission (Table 6, model 1): a glucose level ≥128 mg/dL (hazard ratio [HR], 2.41; 95% CI, 1.11-5.10), systolic blood pressure (HR, 0.97; 95% CI, 0.96-0.99), troponin T (HR, 4.88; 95% CI, 2.66-8.97), and development of heart failure (HR, 1.04; 95% CI, 1.01-1.06). A 10-mg increase in glucose level was associated with an increase in the risk of death of 2.56 (95% CI, 1.12-5.32) (Table 6, model 2).
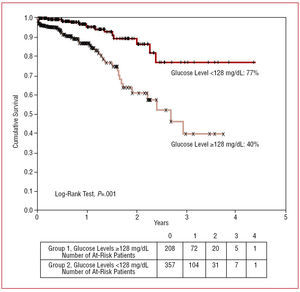
The actuarial survival at 4 years was 40% and 77% in groups 1 and 2, respectively (log-rank tests, P<.001) (Figure 2). Follow-up was completed in 98% of patients, with a mean period of 42 (6) months.
Figure 2. Kaplan-Meier survival curves for groups 1 and 2.
DISCUSSION
In this study, undertaken in an unselected population of patients with acute coronary syndrome admitted to hospital in the 24 hours following the onset of symptoms, glucose levels ≥128 mg/dL, present in more than a third of the population, were associated with an independent increase in the risk of long-term mortality.
Hyperglycemia and Stress
It is known that stress during acute infarction can lead to increased glucose levels in the first few hours.4,5 Excessive secretion of catecholamines causes, in addition to signs and symptoms, an increase in cardiac oxygen consumption due to the release of fatty acids. This favors glycogen breakdown and contributes to an increase in glucose levels that, in association with increased concentrations of glucagon and cortisol, leads to a reduction in glucose tolerance and reduced myocardial contractility. These changes are associated with a greater incidence of deleterious effects such as no-reflow phenomenon3,12,13 and heart failure during the period of hospital admission.5 Stress hyperglycemia may be an indicator of more extensive myocardial damage, which would be reflected in increased concentrations of markers of myocardial necrosis, with or without more extensive coronary disease14,15 and worse prognosis.16-20 The results of this study confirm those findings, given the observed increase in the level of those markers.
Hyperglycemia as a Predictor of Mortality
In this study, we observed that hyperglycemia at admission was associated with a 2.41-fold increase in the risk of mortality in patients with acute coronary syndrome. Most studies have been undertaken in populations of patients with acute ST-segment elevation myocardial infarction, in whom an increase in mortality has been demonstrated along with the development of heart failure, cardiogenic shock, and arrhythmias.5,9 However, this unselected patient series also included non-ST-segment elevation infarctions and high-risk unstable angina. Elevated glucose concentrations have been independently associated with negative outcome.5,20-24 In a previous study, we observed that for each gram per liter increase in the glucose level there was a 1.7-fold increase in the risk of death during follow-up in patients with acute infarction.11 Another interesting observation in this study was that while 27.8% of the total population had a history of diabetes, the definition proposed for hyperglycemia at admission allowed detection of 36.8% of cases, almost 10% higher in the overall population. Nowadays, it is known that recent onset diabetes represents a high risk and that its presence is associated with similar risk to that of previous infarction.25
Patients with hyperglycemia more often suffer heart failure during the period of hospital admission, ref lecting greater impairment of the cardiac muscle, a lower ejection fraction, increased release of markers of myocardial necrosis, and more severe coronary disease.26,27 We believe that it is important to have access to an appropriate parameter for the identification of left ventricular dysfunction at the time of presentation of acute coronary syndrome, as well as for the detection of potentially reversible myocardial dysfunction that may benefit from revascularization; this may facilitate the earliest possible initiation of treatment to prevent remodeling.28
An increased troponin level at admission was shown to be a prognostic marker in patients with or without ST-segment elevation. A metaanalysis of 7 clinical trials and 19 cohort studies showed that patients with positive results for troponin had higher mortality, with a 3-fold to 8-fold increase in the risk of death.29 In patients with ST-segment elevation, increased troponin levels at admission have been associated with increased mortality, as well as with greater development of heart failure and cardiogenic shock at 30-day follow-up.30 The increased mortality may be simply a function of the longer duration of symptoms, the presence of prior silent ischemic events that caused troponin release, or greater myocardial vulnerability.31 In out study, elevated troponin T at admission was a much better prognostic marker than hyperglycemia, with more than 4 times the risk of death during follow-up for every nanogram increase per deciliter.
Hypotension at admission was also a prognostic marker: for each 1 mm Hg reduction in blood pressure the risk increased by 2.2%.
Other studies have shown that hyperglycemia is an indicator of worse long-term outcome.6,7 Bolk et al4 reported an overall mortality of 19.3% at 1-year follow-up in an unselected population of 336 consecutive patients with AMI, and that rate increased to 44% in patients with glucose levels greater than 11.1 mmol/L. In our study, patients with glucose levels ≥128 mg/dL showed worse survival at 4-year follow-up (40% compared with 77%) and if we look carefully at the survival curves we see that they separate initially during the period of hospitalization and that the divergence is more marked after 18 months. This finding indicates that elevated glucose levels at admission allow selection of a group of patients at high clinical risk.
Limitations
Despite showing data from the "real world," the study does not provide information on the treatment provided during hospital stay in patients with hyperglycemia. In addition, glucose level was not monitored periodically during hospital stay. The inclusion of acute coronary syndromes up to 24 hours following onset of symptoms may encompass various initial therapeutic strategies, which may influence long-term prognosis.
Clinical Implications
The results of this study show that in patients with acute coronary syndromes hyperglycemia (cut point, ≥128 mg/dL) allows identification of a group of individuals at high risk of adverse events during follow-up and lower survival at 4 years. The clinical usefulness of these findings should be complemented with the implementation of a diet low in carbohydrates, provision of insulin during the acute event,32-36 as shown in the DIGAMI study,32 and with the use of antithrombotic drugs such as clopidogrel and statins, and invasive strategies such as primary angioplasty, and early revascularization for unstable angina, or non-Q-wave infarction.37-40
It is important to remember that through this simple method, available in any hospital, it is possible to identify at-risk individuals at admission, allowing the best strategies possible to be offered or for the patient to be referred to another hospital.
Other possible changes in the future would consist of early recognition of hyperglycemia and the application of dietary measures and insulin treatment,41,42 since optimization of treatment for diabetes and glucose intolerance could improve cardioprotection and reduce morbidity and mortality in these syndromes.13
CONCLUSIONS
In patients with acute coronary syndrome, hyperglycemia at admission (cut point ≥128 mg/dL) is associated with an increase in long-term risk that, in addition, is a strong independent predictor.
Correspondence: Dra. S.M. Macín.
Unidad de Cuidados Intensivos Coronarios.
Instituto de Cardiología Juana F. Cabral.
Bolívar, 1334. 3400 Corrientes. Argentina.
E-mail: stellam@gigared.com or macinucic@hotmail.com
Manuscript received January 5, 2006.
Accepted for publication September 14, 2006.