The incidence and predictors of recurrent restenosis after drug-coated balloon (DCB) angioplasty for drug-eluting stent (DES) restenosis remain poorly studied. We sought to evaluate the incidence and predictors of recurrent restenosis among participants in randomized controlled trials receiving DCB angioplasty for DES restenosis.
MethodsThe clinical and lesion data of individuals enrolled in 6 randomized controlled trials of DCB angioplasty for DES restenosis were pooled. All patients included in this report were assigned to receive paclitaxel-coated balloon angioplasty with the SeQuent Please DCB (B Braun, Melsungen, Germany). The current analysis focused on participants with available follow-up angiography at 6 to 9 months. The incidence of recurrent restenosis, defined as diameter restenosis ≥ 50% in the in-segment area at follow-up angiography, and its clinical and angiographic predictors were evaluated.
ResultsA total of 546 patients were combined in a single dataset. Angiographic follow-up at 6 to 9 months was available for 484 patients (88.6%) with 518 treated lesions. Recurrent restenosis was detected in 101 (20.8%) patients. On multivariable analysis, lesion length (OR, 1.58; 95%CI, 1.10-2.26; P=.012 for 5mm increase) and vessel size (OR, 1.42; 95%, 1.12-1.79; P=.003 for 0.5mm reduction) were independently associated with recurrent restenosis.
ConclusionsIn the largest cohort to date of individuals with angiographic surveillance after DCB angioplasty for DES restenosis, we demonstrated that recurrent restenosis occurs in approximately 1 out of 5 patients. Predictors of recurrent restenosis are increased lesion length and small vessel size.
Keywords
Contemporary drug-eluting stents (DES) have markedly reduced the need for reintervention compared with both bare metal stents and early-generation DES. However, the occurrence of restenosis due to neointimal proliferation and/or neoatherosclerosis within stented segments is still the main reason for DES failure.1 Moreover, the optimal treatment of DES restenosis remains a matter of debate and continues to be associated with high rates of recurrent restenosis.2
In patients with DES restenosis, European guidelines recommend treatment with either drug-coated balloon (DCB) or repeat stenting with DES; recommendations for both options are supported by a similar level of evidence.3 Drug-coated balloon represents an attractive treatment option, providing antiproliferative efficacy without the requirement for an additional stent implant.4 Although recent studies of patients with DES restenosis ranked the antirestenotic potency of DCB as the second most effective treatment after repeat stenting with everolimus-eluting stents,5 this treatment might be the preferred option for patients due to concerns about the late outcomes of patients treated with multiple stent layers.6
Follow-up angiography is the modality of choice for the detection of lumen renarrowing after coronary intervention and for assessment of device efficacy.7 To date, however, investigations of the incidence and predictors of recurrent restenosis after DCB angioplasty for DES restenosis remain scarce. Moreover, the identification of clinical, angiographic and procedural risk factors predicting the risk of recurrent restenosis at follow-up angiography may provide a basis for treatment optimization or individualization of revascularization strategies in specific patient and lesion subsets. In this report, we evaluated the incidence and predictors of recurrent restenosis in a cohort of patients treated with DCB angioplasty for DES restenosis in the setting of randomized controlled trials.
METHODSData Sources and Eligibility CriteriaFor inclusion in the current analysis, randomized trials of DCB therapy for patients with stable or unstable coronary artery disease because of DES restenosis were identified by searching Medline, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the abstracts of scientific sessions, and relevant websites. No restrictions in terms of language or publication status were imposed. The reference lists from all eligible studies and previous meta-analyses on this topic5,8 were checked to identify further citations. Search terms included the keywords and the corresponding Medical Subject Headings for “drug-coated (-eluting) balloon”, “paclitaxel-coated (-eluting) balloon”, “drug-eluting stent(s)”, “restenosis”, “trial”, and “randomized trial”. Inclusion criteria consisted of randomized design and the availability of follow-up angiography data 6 to 9 months after the index procedure. Investigations of DCB angioplasty for indications other than DES restenosis were ineligible. The last search was performed on June 22, 2016.
Collection of Individual Participant Data and Quality AssessmentTwo investigators (S. Cassese and R.A. Byrne) independently assessed publications for eligibility at the title and/or abstract level. Divergences were resolved by consensus. Studies that met the inclusion criteria were selected for further analysis. Freedom from bias was evaluated for each study in accordance with The Cochrane Collaboration method.9 Composite quality scores were not assigned.10
Of 8 studies identified through the electronic search, 2 randomized trials11 were excluded since the overall percentage of patients receiving DCB angioplasty because of DES restenosis was<5%. Finally, 6 randomized trials12–17 were available for inclusion in the present analysis. The principal investigators of these studies were contacted to provide individual data of participants randomly assigned to DCB angioplasty. Data was transferred without patient identifiers to the ISAResearch Center (Deutsches Herzzentrum Mu¿nchen, Technische Universität München, Munich, Germany) and combined in a single pooled database. The final dataset was checked for completeness and consistency and compared with the results of prior publications. Principal investigators were directly contacted in case of requirement for additional data. Data were analyzed according to the intention-to-treat principle. Each study included in the present analysis was approved by the institutional review board or ethics committee at each participating center, and all patients provided informed, written consent before receiving the assigned treatment.
Angiographic Data and Study DefinitionsAt baseline, procedural parameters were gathered and follow-up coronary angiograms were digitally recorded and assessed off-line with automated edge-detection systems by independent operators in all studies.12–17 Lesion characteristics were described in accordance with standard definitions, while restenosis morphology was classified according to criteria modified from Mehran et al.18
The angiographic and procedural parameters collected for the current analysis were vessel size, lesion length, initial diameter stenosis, maximal balloon pressure, final lumen diameter, and final diameter stenosis. The balloon-to-vessel ratio was calculated with the maximal diameter of the inflated balloon divided by the coronary vessel size. The percentage of diameter stenosis was calculated as ([1-minimal lumen diameter/reference vessel diameter] × 100). Restenosis (angiographic or binary), the main outcome of interest in this report, was defined as diameter stenosis ≥ 50% in the in-segment area (defined as the balloon-treated area and 5-mm segments proximal and distal to the treated area).
Data Synthesis and Statistical AnalysisCategorical data are presented as counts and proportions (percent). Continuous data are presented as median and interquartile range [25th to 75th percentiles] or as mean±standard deviation, as appropriate. The data distribution was tested for normality using the Kolmogorov-Smirnov test. For patient-level data, the differences between groups were checked for significance using the Student t or Kruskal-Wallis tests (continuous data) or the chi-squared or Fisher exact tests where the expected cell value was<5 (categorical variables). For lesion-level data, the differences between groups were checked for statistical significance using generalized estimating equations (R-package gee) to address the intrapatient correlation in patients with multilesion interventions. Depending on the nature of the dependent variable, we used the Gaussian or the binomial family for continuous and discrete variables, respectively. We employed the exchangeable link function. The predictors of recurrent restenosis were studied by means of a multivariable analysis. Missing baseline data were imputed by using the predictive mean matching function (R-package mice) and the selection of variables for the multivariable model was performed by the use of the LASSO (Least Absolute Shrinkage and Selection Operator) regression method after entering all baseline and procedural characteristics as candidates (R-package glmnet). Finally, a logistic regression model stratified by trial was applied for recurrent restenosis after entering also a cluster term to account for the presence of multiple treated lesions in the same patient. The adjusted odds ratios (ORs) with 95% confidence intervals (95%CI) were used as summary statistics. A P-value of<.05 was considered significant. All analyses were performed in R (version 3.3.2, R Foundation for Statistical Computing, Vienna, Austria). This study was reported in compliance with the Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data (Table 1 of thesupplementary material).19
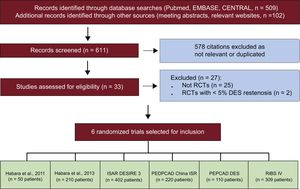
RESULTSFigure 1 shows the flow diagram for the trial selection process, while Table 1 displays the main features of selected studies. Briefly, patients with clinical or instrumental evidence of stable or unstable coronary artery disease and ≥ 50% diameter stenosis in a previous DES-treated segment were included in 6 randomized trials.12–17 Drug-coated balloon therapy consisted of a balloon angioplasty with the SeQuent Please catheter (B Braun, Melsungen, Germany). The coating of the DCB under investigation comprises of the antirestenotic drug paclitaxel at a dose of 3μg for mm2 of balloon surface and contrast medium (iopromide) as drug-vehicle. The control therapy was conventional balloon angioplasty12–15 or repeat stenting with DES.15–17 In all trials except one14 the primary endpoint consisted of angiographic measures of efficacy after 6 to 9 months. Of note, the participants of the trial with a clinical primary endpoint accounted for 9.7% of the entire cohort available for this report. Aspirin at the time of the index intervention and a loading dose of platelet adenosine-diphosphate receptor inhibitors were administered in all patients. Anticoagulation during coronary interventions was accomplished through administration of either unfractionated heparin or bivalirudin. At discharge, aspirin therapy was recommended indefinitely for all participants, while platelet adenosine-diphosphate receptor inhibitors were prescribed for a period of time ranging from 3 to 12 months, depending on clinical presentation or protocol-specific requirements. The evaluation of risk of bias among studies is reported in Table 2 of the supplementary material.
Flow diagram of trial selection process. CENTRAL, Cochrane Central Register of Controlled Trials; ISAR-DESIRE 3, Randomized Trial of Paclitaxel-Eluting Balloon, Paclitaxel-Eluting Stent and Plain Balloon Angioplasty for Restenosis in “-Limus”-Eluting Coronary Stents; PEPCAD China ISR, A Multicenter, Randomized, Active Controlled Clinical Study to Evaluate the Safety and Efficacy of the Treatment of In-stent Restenosis Lesion by Paclitaxel-eluting PTCA-Balloon Catheter vs Paclitaxel-eluting Stent; PEPCAD DES, Treatment of DES-In-Stent Restenosis With SeQuent Please Paclitaxel Eluting PTCA Catheter; RCT, randomized controlled trial; RIBS IV, Restenosis Intrastent of Drug-eluting Stents: Paclitaxel-eluting Balloon vs Everolimus-eluting Stent). A Prospective, Multicenter and Randomized Clinical Trial.
Main Features of Trials Selected for Inclusion in the Study
| Trial | Enrollment period | Patients included, no. | Design | Main inclusion criteria | Main exclusion criteria | Primary endpoint |
|---|---|---|---|---|---|---|
| Habara et al.12 | 2008-2009 | 50 | Single-center, single-blind, RCT of DCB vs POBA (1:1 ratio) for DES restenosis | • Age ≥ 18 y • Stable CAD • First DES restenosis (SES restenosis) • RVD ≥ 2.5 and ≤ 3.5 mm • Lesion length<26 mm | • ACS • Severe CKD • Prior stenting<6 mo • DES restenosis involving uLMCA, ostial lesion • Bifurcation lesion • CTO | 6 mo LLL |
| Habara et al.14 | 2009-2011 | 210 | Multicenter, open-label, RCT of DCB vs POBA (2:1 ratio) for DES or BMS restenosis | • Age>20 y • Stable or unstable CAD • Single BMS or DES restenosis (EES, SES, ZES restenosis) lesion or ≤ 2 restenotic lesions in different vessels • RVD ≥ 2.0 and ≤ 4.0 mm • Lesion length ≤ 22 mm | • <30% LVEF • AMI<72 h • CKD • Any PCI<28 d • PCI with DES<6 mo • Any CVA<6 mo • Antiplatelet, anticoagulant, paclitaxel or contrast media intolerance • Pregnancy or childbearing potential • Severe illnesses with<12 mo life expectancy • >50% uLMCA diameter stenosis • Tortuous (> 90° angle) lesion • Multiple lesions in the target vessel • CTO, heavily calcified lesion, DES restenosis involving bypass graft or bifurcation with>2.0mm RVD of side branch | 6 mo TVF (cardiac death related to the target vessel, MI and TVR) |
| ISAR-DESIRE 315 | 2009-2011 | 402 | Multicenter, open-label, RCT of DCB vs PES vs POBA (1:1:1 ratio) for DES restenosis | • Age>18 y • Stable or unstable CAD • >50% diameter DES restenosis (BES, EES, SES, ZES restenosis) | • STEMI<48 h • Cardiogenic shock • Severe CKD • Severe illnesses with<12 mo life expectancy or that might result in protocol noncompliance • Contraindications or known allergy to antiplatelet therapy or paclitaxel, pregnancy or childbearing potential • DES restenosis involving uLMCA or bypass graft | 6-8 mo diameter stenosis |
| PEPCAD China ISR16 | 2011-2012 | 220 | Single-center, single-blind, RCT of DCB vs PES (1:1 ratio) for DES restenosis | • Age ≥ 18 to ≤ 80 y • Stable or unstable CAD • >50% and ≤ 70% diameter DES restenosis (EES, SES, PES restenosis) • ISR Mehran class I-III • RVD ≥ 2.5 and ≤ 4.0 mm • Lesion length ≤ 30 mm | • AMI<1 wk • Heart failure NYHA class IV • Severe valvular heart disease • CVA<6 mo • Severe CKD • DES restenosis involving bifurcation with>2.5mm RVD of side branch • Thrombus in the target vessel | 9 mo LLL |
| PEPCAD DES13 | 2009-2011 | 110 | Multicenter, single-blind, RCT of DCB vs POBA (2:1 ratio) for DES restenosis | • DES restenosis (EES, SES, PES restenosis) • RVD ≥ 2.5 and ≤ 3.5 mm • Lesion length<22 mm | • Planned surgery within 6 mo • Contraindication to antiplatelet therapy • Thrombus in the target vessel • DES restenosis involving uLMCA or bypass graft • Bifurcation lesion • Ostial lesion • Multiple lesions in the target vessel • CTO | 6 mo LLL |
| RIBS IV17 | 2010-2013 | 309 | Multicenter, open-label, RCT of DCB vs EES (1:1 ratio) for DES restenosis | • Stable or unstable CAD • >50% diameter DES restenosis • RVD>2.0 mm • Lesion length ≤ 30 mm | • CTO • < 1 mo DES restenosis • AMI • Thrombus in the target vessel • Severe peripheral vascular disease • Severe illnesses with<12-mo life expectancy or that might result in protocol noncompliance • Contraindications to antiplatelet therapy | 6-9 mo MLD |
ACS, acute coronary syndrome; AMI; acute myocardial infarction; BES, biolimus-eluting stent; BMS, bare metal stent; CAD, coronary artery disease; CKD, chronic kidney disease; CTO, chronic total occlusion; CVA, cerebrovascular accident; DCB, drug-coated balloon; DES, drug-eluting stent; EES, everolimus-eluting stent; ISAR-DESIRE 3, Randomized Trial of Paclitaxel-Eluting Balloon, Paclitaxel-Eluting Stent and Plain Balloon Angioplasty for Restenosis in “-Limus”-Eluting Coronary Stents; ISR, in-stent restenosis; LLL, late lumen loss; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MLD, minimal lumen diameter; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PEPCAD China ISR, A Multicenter, Randomized, Active Controlled Clinical Study to Evaluate the Safety and Efficacy of the Treatment of In-stent Restenosis Lesion by Paclitaxel-eluting PTCA-Balloon Catheter vs Paclitaxel-eluting Stent; PEPCAD DES, Treatment of DES-In-Stent Restenosis With SeQuent Please Paclitaxel Eluting PTCA Catheter; PES, paclitaxel-eluting stent; POBA, plain old balloon angioplasty; RCT, randomized controlled trial; RIBS IV, Restenosis Intrastent of Drug-eluting Stents: Paclitaxel-eluting Balloon vs Everolimus-eluting Stent). A Prospective, Multicenter and Randomized Clinical Tria; RVD, reference vessel diameter; SES, sirolimus-eluting stent; STEMI, ST-segment elevation myocardial infarction; TVF, target vessel failure; TVR, target vessel revascularization; uLMCA, unprotected left main coronary artery; ZES, zotarolimus-eluting stent.
Among selected trials,12–17 546 individuals with 588 lesions were assigned to DCB therapy because of DES restenosis. Table 3 of the supplementary material displays the main features of patients randomly assigned to DCB angioplasty in each individual trial. Among participants, a total of 484 individuals (88.6%) with 518 treated lesions had angiographic surveillance available at a median of 202 days (range, 180-275) after the index procedure. At this time point, recurrent restenosis was observed in 101 patients (20.8%) with 103 treated lesions. The study flow diagram is displayed in the Figure of the supplementary material.
A total of 62 patients did not undergo follow-up angiography: these patients were slightly older (age 62.2 [58.0-68.0] vs 59.0 [52.1-67.5] years; P=.09), were more likely to have a diagnosis of diabetes (58.1% vs 43.0%; P=.02), and had similar rates of previous myocardial infarction (43.5% vs 46.9%; P=.62) in comparison to patients with follow-up angiography. Four out of 62 patients died before the scheduled angiography.
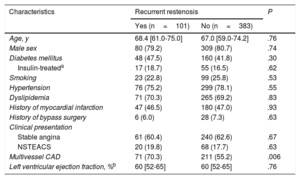
Univariable AnalysisThe baseline clinical characteristics of patients with and without recurrent restenosis at follow-up angiography are shown in Table 2. The proportions of individuals with diabetes mellitus and with a history of myocardial infarction approached 50% and were comparable between groups. In both groups, most patients complained of stable angina at the time of the index intervention. Multivessel coronary artery disease was more likely to be present in patients with recurrent stenosis than in those without.
Baseline Clinical Features of Patients With Available Follow-up Angiography After Drug-coated Balloon Angioplasty for Drug-eluting Stent Restenosis
| Characteristics | Recurrent restenosis | P | |
|---|---|---|---|
| Yes (n=101) | No (n=383) | ||
| Age, y | 68.4 [61.0-75.0] | 67.0 [59.0-74.2] | .76 |
| Male sex | 80 (79.2) | 309 (80.7) | .74 |
| Diabetes mellitus | 48 (47.5) | 160 (41.8) | .30 |
| Insulin-treateda | 17 (18.7) | 55 (16.5) | .62 |
| Smoking | 23 (22.8) | 99 (25.8) | .53 |
| Hypertension | 76 (75.2) | 299 (78.1) | .55 |
| Dyslipidemia | 71 (70.3) | 265 (69.2) | .83 |
| History of myocardial infarction | 47 (46.5) | 180 (47.0) | .93 |
| History of bypass surgery | 6 (6.0) | 28 (7.3) | .63 |
| Clinical presentation | |||
| Stable angina | 61 (60.4) | 240 (62.6) | .67 |
| NSTEACS | 20 (19.8) | 68 (17.7) | .63 |
| Multivessel CAD | 71 (70.3) | 211 (55.2) | .006 |
| Left ventricular ejection fraction, %b | 60 [52-65] | 60 [52-65] | .76 |
CAD, coronary artery disease; NSTEACS, non—ST-segment elevation acute coronary syndrome.
Data are presented as median [25th-75th percentiles] or counts (proportion) of patients.
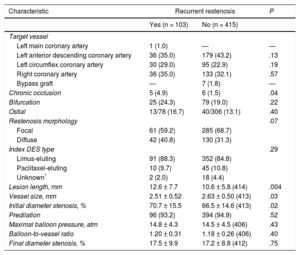
Baseline angiographic and procedural characteristics of lesions with and without recurrent restenosis are shown in Table 3. Treatment of chronic occlusions, long lesions, small vessels and higher-degree of baseline stenosis was more likely in patients with recurrent stenosis than in those without. There was a numerically higher proportion of diffuse-pattern restenosis in those lesions showing recurrent restenosis at follow-up angiography than in those that did not. Notably in both groups, more than 4 out of 5 of restenotic stents eluted sirolimus or its analogs.
Angiographic and Procedural Features of Patients With Available Follow-up Angiography After Drug-coated Balloon Angioplasty for Drug-eluting Stent restenosis
| Characteristic | Recurrent restenosis | P | |
|---|---|---|---|
| Yes (n = 103) | No (n = 415) | ||
| Target vessel | |||
| Left main coronary artery | 1 (1.0) | — | — |
| Left anterior descending coronary artery | 36 (35.0) | 179 (43.2) | .13 |
| Left circumflex coronary artery | 30 (29.0) | 95 (22.9) | .19 |
| Right coronary artery | 36 (35.0) | 133 (32.1) | .57 |
| Bypass graft | — | 7 (1.8) | — |
| Chronic occlusion | 5 (4.9) | 6 (1.5) | .04 |
| Bifurcation | 25 (24.3) | 79 (19.0) | .22 |
| Ostial | 13/78 (16.7) | 40/306 (13.1) | .40 |
| Restenosis morphology | .07 | ||
| Focal | 61 (59.2) | 285 (68.7) | |
| Diffuse | 42 (40.8) | 130 (31.3) | |
| Index DES type | .29 | ||
| Limus-eluting | 91 (88.3) | 352 (84.8) | |
| Paclitaxel-eluting | 10 (9.7) | 45 (10.8) | |
| Unknown* | 2 (2.0) | 18 (4.4) | |
| Lesion length, mm | 12.6 ± 7.7 | 10.6 ± 5.8 (414) | .004 |
| Vessel size, mm | 2.51 ± 0.52 | 2.63 ± 0.50 (413) | .03 |
| Initial diameter stenosis, % | 70.7 ± 15.5 | 66.5 ± 14.6 (413) | .02 |
| Predilation | 96 (93.2) | 394 (94.9) | .52 |
| Maximal balloon pressure, atm | 14.8 ± 4.3 | 14.5 ± 4.5 (406) | .43 |
| Balloon-to-vessel ratio | 1.20 ± 0.31 | 1.18 ± 0.26 (406) | .40 |
| Final diameter stenosis, % | 17.5 ± 9.9 | 17.2 ± 8.8 (412) | .75 |
DES, drug-eluting stent.
Data are presented mean±standard deviation or counts (proportion) of lesions. Denominators have been provided when they differ from the total number of lesions.
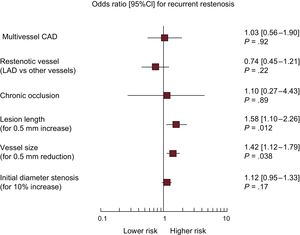
Figure 2 shows the plot of ORs for the variables entered in the final model. The presence of longer lesion length (OR, 1.58; 95%CI, 1.10-2.26; P=.012 for 5mm increase) and smaller vessel size (OR, 1.42; 95%CI, 1.12-1.79; P=.003 for 0.5mm reduction) were independently associated with a higher likelihood of recurrent restenosis
Multivariable analysis for recurrent restenosis in patients treated with drug-coated balloon angioplasty for drug-eluting stent restenosis. Plot of odds ratios associated with recurrent restenosis stratified by trial. The squares indicate the point estimate and the left and the right ends of the lines the [95%CI]. 95%CI, 95% confidence interval; CAD, coronary artery disease; LAD, left anterior descending coronary artery.
In the current analysis, we investigated the incidence and predictors of recurrent restenosis at surveillance angiography in 546 individuals treated with DCB angioplasty for DES restenosis across 6 randomized trials. The dataset analyzed represents the largest population with follow-up angiography after DCB angioplasty for DES restenosis studied so far. The main findings are the following: a) recurrent restenosis after DCB angioplasty for DES restenosis occurs in approximately 1 out of 5 patients; b) the presence of longer lesion length and smaller vessel size are predictors of recurrent restenosis.
The continuous iteration of DES therapy has expanded the indications of percutaneous interventions to increasingly complex patient and lesion populations.1 Accordingly, as rates of stent failure may be considered a function of disease complexity,20 the absolute number of patients presenting with DES restenosis remains considerable in absolute terms and is expected to increase in the years to come. Since DCB angioplasty is among the most effective options for patients with DES restenosis,5 investigations of recurrent restenosis in this setting represent an important undertaking.
The present report investigated the largest dataset of participants randomly assigned to receive DCB angioplasty for DES restenosis across controlled clinical trials. Notably, baseline features were comparable to those of individuals with DES restenosis receiving DCB therapy in daily practice.21,22 We found that recurrent binary angiographic restenosis occurs in nearly 21% of patients. These results are broadly consistent with those from individual clinical trials and support DCB as the second most efficacious treatment for DES restenosis after repeat everolimus-eluting stent implantation, which has been associated with an incidence of recurrent restenosis as high as 11.3%.23
In this analysis, long lesions were strong predictors of recurrent restenosis after DCB angioplasty for DES restenosis. In particular, we found a 58% higher risk of recurrence for each increase in lesion length by 5mm. Proper contact with the underlying restenotic tissue of sufficient drug concentrations is a prerequisite for the efficacy of DCB therapy.4 In this respect, a higher displacement of drug particles from balloon catheters into the bloodstream during delivery of the balloon in long restenotic segments may contribute to a reduced DCB efficacy in this setting.24 In addition, other factors associated with poor outcomes, such as stent underexpansion, are more frequent with long-segment stenting25 and likely impair an effective drug transfer to the vessel wall.
Small-caliber coronary arteries remain the factor most closely correlated with restenosis regardless of the type of percutaneous treatment.26 Intuitively, at an individual patient level, for any given degree of neointimal overgrowth after intervention the probability that the resultant diameter stenosis exceeds 50% is a function of vessel size and residual after procedural stenosis. Here, we report a 42% higher risk of recurrent restenosis for each 0.5mm decrease in reference vessel diameter. On the one hand, DCB represents an attractive treatment option among patients with DES restenosis of small coronary vessels, by implementing an antiproliferative therapy by means of a nonstent-based platform, thus avoiding a mechanical shrinkage of the vessel lumen due to an additional metal layer. On the other hand, the superior antirestenotic efficacy of alternative treatments, namely contemporary high-performance stent platforms such as everolimus-eluting stents, may be of particular relevance for patients with DES restenosis of small coronary vessels.26
It is interesting to note that in our analysis the type of underlying DES did not emerge as an independent predictor of recurrent restenosis after DCB angioplasty for DES restenosis. Although this observation was based on a limited number of patients, it is in keeping with data from previous studies.27 Current commercially available DCB catheters use exclusively the taxol-derivative paclitaxel as antirestenotic drug.4 Although the influence of drug-resistance on DCB efficacy in patients with DES restenosis is of scientific interest, paclitaxel-eluting stents are no longer used in daily practice and limus-based DCB catheters are still in the development phase.28 In this regard, it remains to be addressed whether the continuous development of new DCB catheters (paclitaxel- and limus-based), the synergy of different technologies (cutting/scoring balloon catheters for predilation before DCB angioplasty), and a more liberal use of intravascular imaging to optimize lesion preparation will decrease the risk of recurrence after DCB angioplasty for DES restenosis.
LimitationsThe current analysis has some limitations. First, it applies only to patients with DES restenosis and the incidence and predictors of recurrence after DCB angioplasty for restenosis of bare metal stents cannot be extrapolated from this study. Second, the rate of recurrent restenosis after DCB angioplasty for DES restenosis at a time point other than 6 to 9 months remains poorly studied and the predictors identified in this report may not apply to patients with recurrent restenosis at a different time point. Third, adjudication of restenosis was based on the percentage diameter of lumen renarrowing at follow-up angiography. The association between further lesion-specific factors (such as restenotic tissue characterization) on subsequent angiographic results was not assessed. Fourth, it should be noted that this study pooled well-selected patients and lesions. In this respect, the recurrence rate rate observed in the present analysis should be interpreted with caution and is not generalizable to higher-risk subsets of patients. Fifth, the original trials included in this report did not routinely assess ischemic burden in patients with recurrent restenosis at 6 to 9 months; in this regard, the clinical relevance of angiographic findings cannot be assessed in this context. Finally, all patients included in this report received DCB angioplasty with a single balloon catheter. Although this aspect eliminates an important source of variability, the available evidence does not support a class effect for DCB platforms.4 In this respect, the angiographic data observed in this analysis should not be extrapolated to other DCB devices.
CONCLUSIONSIn the largest cohort of patients with angiographic surveillance after DCB angioplasty for DES restenosis, recurrence was found in approximately 1 out of 5 patients. Independent predictors of recurrent restenosis were increased lesion length and small vessel size.
CONFLICTS OF INTERESTA. Kastrati reports patent applications related to drug-eluting stent technologies. R.A. Byrne reports receiving lecture fees from B. Braun Melsungen AG, Biotronik and Boston Scientific and scientific support from Boston Scientific and Heartflow. M. Waliszewski is a full-time employee of the Medical Scientific Affairs Department of B. Braun Melsungen AG.
- –
The occurrence of restenosis within stented segments still represents the principal reason of failure after contemporary DES therapy.
- –
Drug-coated balloon is an attractive therapeutic option by providing antiproliferative efficacy without requirement for an additional stent.
- –
Nevertheless, the treatment of DES restenosis continues to be associated with high recurrence rates.
- –
In this pooled analysis of individual participant data from 6 randomized controlled trials of drug-coated balloon angioplasty for DES restenosis, we demonstrate that recurrent restenosis at follow-up angiography occurs in approximately 1 out of 5 patients.
- –
Predictors of recurrent restenosis are increased lesion length and small vessel size.
- –
Knowledge of the incidence and predictors of recurrent restenosis after drug-coated balloon angioplasty for DES restenosis may provide a basis for treatment optimization or individualization of revascularization strategies in specific patient and lesion subsets.



![Multivariable analysis for recurrent restenosis in patients treated with drug-coated balloon angioplasty for drug-eluting stent restenosis. Plot of odds ratios associated with recurrent restenosis stratified by trial. The squares indicate the point estimate and the left and the right ends of the lines the [95%CI]. 95%CI, 95% confidence interval; CAD, coronary artery disease; LAD, left anterior descending coronary artery. Multivariable analysis for recurrent restenosis in patients treated with drug-coated balloon angioplasty for drug-eluting stent restenosis. Plot of odds ratios associated with recurrent restenosis stratified by trial. The squares indicate the point estimate and the left and the right ends of the lines the [95%CI]. 95%CI, 95% confidence interval; CAD, coronary artery disease; LAD, left anterior descending coronary artery.](https://static.elsevier.es/multimedia/18855857/0000007100000008/v1_201808061038/S1885585717303936/v1_201808061038/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6IlBPKzhFcFE5SDQ5NGpuVHVxTHdEU3c9PSIsInZhbHVlIjoiOWNYMnM1cGJuZ2hNMjhldWxJbHRyd0NPRU5tQTllMFZFeUtuc2xCWk5oVT0iLCJtYWMiOiIxZTQ1MGJkYjk4ZWNjZmU0ZTY5NWFmOTIwYjA4YTIyOTcxMDkyZDM5MmJjZTUwMTYxMjEzYzg0NTlkZmI0ZjBiIiwidGFnIjoiIn0=)